
INTRODUCTION OF
THE RESEARCH DEPARTMENT
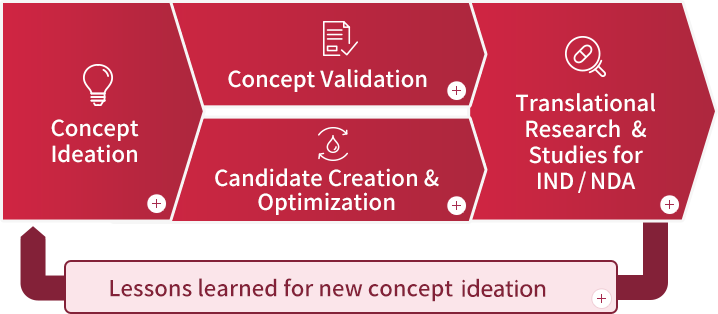
Drug development candidates are created through multiple stages of drug discovery research. At each of these stages, members with various specialties are active in their respective roles.
(Click on the following figures to see the tasks and roles at each stage.)

・IND: Investigational New Drug
・NDA: New Drug Application

Concept Ideation
Drug discovery research is initiated with the refinement of drug discovery ideas and the creation of drug discovery concepts.
We focus on biology with disease-relevance, such as biomolecules and physiological mechanisms, and use literature surveys and various databases to identify drug targets.
Overview
Research to find new “drug seeds”
Although therapeutic agents have already been developed and marketed for many diseases, there are still many diseases for which there are no satisfactory treatments. There are also diseases for which new drugs are strongly needed due to inadequate efficacy or strong side effects, even for those diseases for which drugs are already available.
In the Concept Ideation stage, we estimate the causes of diseases and symptoms based on changes at the individual, cellular, and molecular levels that underlie the diseases, and formulate and verify methods (drug discovery concepts) to improve the conditions of the patients based on this information.
Drug discovery concepts are developed based not only on the results of in-house research, but also on findings from joint research with academia and other institutions, as well as publications from external organizations. The drug discovery concept is then tested for efficacy against the disease at the individual and cellular levels using tool compounds, antibodies, and nucleic acids.
Drug discovery concepts that have been shown to be effective in disease models and cells proceed to the Candidate Creation & Optimization stage, where they are transformed into a form (small molecule compound, antibody, nucleic acid, cell, etc.) to be developed as a drug, and optimization studies are conducted.

Concept Validation
It's a research stage to confirm the validity of drug discovery concepts.
Based on the drug discovery concept, we establish evaluation methods for the desired biological responses and physiological functions, and create an appropriate screening cascade to narrow down development candidates with the desired efficacy and safety profile.
Employee Q&A
 Pharmacology Researcher
Pharmacology ResearcherGraduated from the Department of Pharmacy, Faculty of Pharmaceutical Sciences
Jun Miyanohara
What are your current work roles and responsibilities?
I am responsible for demonstrating at the non-clinical level the drug discovery concept of a candidate product selected through screening in Candidate Creation & Optimization.
I am required to build compelling data sets and stories that demonstrate the potential to deliver new value to patients that cannot be delivered by existing drugs or competing products.
It is important, of course, that a candidate product demonstrate efficacy in a pathological model, but at the same time, it is important to clarify its mechanism of action to the best of our ability. I believe that this validation has three major strategic implications.
The first is competitive advantage. Based on the proven mechanism of action, you will be able to theoretically explain how the drug adds value to existing drugs or competitive products.
The second is safety. If a toxicity finding is observed in a safety study conducted in collaboration with experts, it is useful to consider whether or not the toxicity is avoidable and independent of the mechanism of action.
Third, patient stratification. The possible mechanism of action is the basis for discussion of which patient population to target. This process is often a part of translational research, for example, pharmacological studies using patient samples to verify in a non-clinical setting whether the drug is expected to work in humans.
What are challenging, interesting, or difficult aspects of your role?
As a person in charge of a program in the final stages of early drug discovery, I often find it difficult to communicate my ideas correctly, especially in a global setting, as I have to collaborate with various parties. For example, I have bi-weekly meetings with overseas development, commercial, and other functions, and I have a hard time preparing materials and having dialogues in English, which is never my forte.
Even so, I sometimes feel that the team's viewpoints are aligned and new issues or necessary actions come to mind only when I hit them with a message I have thought through in my own way and discussed it with them. The moment I feel that I am contributing to team building and promotion of the program is a very rewarding moment.
In addition, as a pharmacological researcher, I feel very fortunate to be in an environment where I am exposed to new science and data on a daily basis. I will never forget the impact I had when we demonstrated the strong efficacy of a hypothetical mechanism of action in an animal model. I believe that the real thrill of being a drug discovery researcher is being able to translate my intellectual desire for science and expertise into value for the world.
Employee Q&A
 Non-clinical Safety Researcher
Non-clinical Safety ResearcherGraduated from Department of Laboratory Technology and Science, School of Medicine and Health Sciences
Kanako Mori
What are your current work roles and responsibilities?
As a non-clinical safety expert, I am responsible for presenting scientific evidence that may affect patient safety at an early stage, after the drug concept has been developed.
Astellas brings cutting-edge science to drug discovery. The most important thing is not whether a treatment is advanced or not, but that patients feel good about receiving it. To achieve this, it is essential to take into account not only efficacy but also safety.
The words of the 16th century physician and chemist Paracelsus “Everything is poison, and nothing is not; only the dose determines what is not poison” sum up the essence of toxicology well. Safety researchers conduct studies that examine all biological reactions in detail and their doses, and interpret the results obtained from various perspectives, including pharmacological effects and pharmacokinetics. I am the profiler who identifies the optimal balance between risk and benefit.
Early in the drug discovery process, I conduct studies to identify critical safety profiles that will have a significant impact on the success of the project. Planning a cascade to select drug seeds from multiple candidates is also essential to establish a safety evaluation system that utilizes new science and technology. Because the speed of drug discovery is extremely fast, we must anticipate the flow of drug discovery and perform optimal evaluations.
What are challenging, interesting, or difficult aspects of your role?
Safety profiling requires a variety of expertise, and while it is important to be familiar with all of them, in practice it is important to have a team activity composed of experts. We constantly consider how to leverage indivisual expertise, work together as a team, and create impactful outocomes. In many cases, we use our expertise in the field of toxicology to develop new evaluation systems using state-of-the-art technology.
On the other hand, the reality is that resources such as time and money are limited in the early stages of drug discovery. In addition, in recent years, the 3Rs* in animals has been attracting attention as a social issue. Drug discovery research needs to be conducted with social issues as well as its own issues in mind, and I sometimes struggle with how to design safety evaluation strategies, but at the same time, I see this as a great opportunity to promote scientific innovation.
As an expert in reproductive and developmental toxicology, I am working on a new study that does not rely on animal experiments. This research has resulted in published results as a consortium study (see also here) and has served as the basis for proposing a new regulatory framework to demonstrate safety. By developing and introducing new evaluation methods, we aim to achieve more effective and reliable safety assessments and further advance the drug discovery process.
*3Rs; Refinement, Reduction, Replacement
Kanako Mori's daily schedule
| 8:30 |
Commuting, arriving at the office My day starts with checking emails, chats, and internal social networking sites and bulletin boards. Within the company, multiple communication tools allow us to exchange information, and I use them according to the situation and purpose. |
| 9:00 | Start of experiment My research focues on reproductive developmental toxicity, and the moment the experiment starts, my priority is to follow the developmental process of the fertilized egg/embryo above all else. |
| 10:00 | While observing the fertilized eggs, I also do desk work such as checking e-mails at the same time. |
| 11:00 | I check the number of samples that can be collected, determine if the experiment can be carried out as planned, and prepare an experiment for the afternoon. |
| 12:00 | Lunch I enjoy a completely off time at the cafeteria rihgt next to office space, having casual conversations with may collegues. |
| 13:00 |
Experiments Experiments are usually conducted from 9am to 3pm. During this time, e-mails and other messages may not be responded to in a timely manner. However, all experiment and meeting schedules are shared with colleagues, so it is easy to focus on my work. |
| 15:00 | Completion of experiment: data compilation, experiment plan |
| 16:00 | Internal meetings and desk work I set up my daily schedule to inculede focus time for not only meetings but also for tasks with approaching deadlines and bainstorming research ideas. |
| 18:00 | Clean up the desk, end of the workday Since the office has a free adress system, I always clean up my desk at the end of the day. Even those who are not good at tidying up will quickly get used to it! |
| 19:00 | After work I participate in Astellas badminton club practice 1-2 times a week. We can also have a community outside of work. |
Candidate Creation & Optimization
It's a research stage to design and optimize development candidates.
Prototype substances are designed based on information collected through screening or other sources, and then modified and optimized into development candidates.
Recent advances in AI technology and dramatic improvements in robotics and computer performance have had a significant impact on the drug discovery process. AI and machine learning using accumulated evaluation data enables efficient design of development candidates, while robots and computers enable efficient evaluation and analysis.
Employee Q&A
 Biologics Researcher
Biologics ResearcherGraduated from the Department of Pharmacy, Faculty of Pharmacy
Rika Yamazaki
What are your current work roles and responsibilities?
I am primarily responsible for creating the development candidates themselves in antibody drug discovery by discovering and optimizing the “Drug seeds”.
Astellas has multiple drug modalities such as cell therapy and targeted proteolysis inducers. We are leveraging this strength to engage in modified antibody drug discovery that crosses with other modalities, in addition to conventional antibody drug discovery. Specific research activities include the construction of screening systems to obtain antibodies with desired profiles, acquisition of antibodies through animal immunization, in vitro and in vivo experiments to evaluate antibody activity, and the construction of in silico antibody design platforms utilizing AI and machine learning.
At the same time, as experts in antibody drug discovery, we are also involved in various stages of the drug discovery research process. For example, you may be involved in theme generation, designing and providing molecules for concept validation, contributing to the validation of drug concepts, or participating in Translational Research & Studies or IND/NDA stage projects to conduct non-clinical studies of development candidates. Some are also responsible for the non-clinical testing of development candidates.
What are challenging, interesting, or difficult aspects of your role?
I find it challenging to refine “proteins” into “biologics” that exhibit the expected drug effects and physical properties through my molecular design. Since I design the structure of the development candidate itself, I am motivated by the feeling that I am involved in important work to deliver new drugs to patients who are waiting for novel therapies.
Prototype proteins rarely have ideal properties from the start. Therefore, we proceed with molecular optimization by repeating the DMTA (Design-Make-Test-Analyze) cycle many times based on experimental data and literature information.
My specialty is protein science, and I am confident in protein preparation and evaluation, but when I first joined the company, I had no experience in improving profiles such as drug efficacy and physical properties as a drug. Nevertheless, my enthusiasm for the challenge of molecular design was appreciated, and I was put in charge of improving the profile of a candidate molecule for a particular research theme. I worked out a design proposal while seeking feedback from experienced researchers, and proceeded with the evaluation from the preparation of the modified form by taking advantage of my own strengths. Once I thought I had succeeded in creating a molecule that met the criteria, I encountered some difficulties, such as the need to redesign the molecule after receiving evaluation results from a newly constructed higher-order evaluation system. However, after repeated and persistent studies, we finally succeeded in creating a molecule with a good profile, which gave us confidence.
Employee Q&A
 Pharmacology Researcher
Pharmacology ResearcherGraduated from the School of Life Science and Technology, Department of Life Science and Technology
Eri Yamashita
What are your current work roles and responsibilities?
In this stage, we are responsible for identifying development candidate from the “drug seeds” which is newly discovered in the Concept Ideation stage. Seeds of medicine are still in the rough in terms of pharmacodynamics, pharmacokinetics and physical properties. We optimize them into the the development candidate, which is more effective and safer for patients.Our works as pharmacology researchers are 1)in vitro/in vivo drug screening based on the knowledge obtained from Concept Validation stage, 2)evaluation of drug efficacy in pathological models that reflect the biology of the disease, etc. The results are shared and discussed with specialists in pharmacokinetics, medicinal chemistry, safety, etc. and utilized in the design of the next candidate product. For small molecules, by continuing the cycle of synthesis-evaluation-discussion, we select only one development candidate out of thousands of compounds.
What are challenging, interesting, or difficult aspects of your role?
There are many rewarding and interesting aspects to working as a pharmacologist in this stage. For example, when we find a drug candidate that shows outstanding efficacy among others. When we are able to construct a better screening system that can predict the efficacy in a pathological model. When the team is planning the next strategy based on the obtained data.... Especially, I find it rewarding to create a development candidate with our own hands, which is the result of every step we take.
I chose this job because I want to contribute to society through the research I love. I am working every day with the image that our products will help patients all over the world.
Of course, in the process, there are many times when optimization does not proceed as expected, and there are also times when trouble occurs in the screening system. I believe that this is not routine work, but work that requires an observant eye and a desire for improvement.
Eri Yamashita's daily schedule
Daily schedule including remote working
| 8:00 (Office work) | Experiment (Create disease model) | Office work |
| 11:00 (Office work) | Meeting related to new themes | |
| 12:00 | Go home, take a break | |
| 13:30 (Work from home) | Edit the minutes of meeting | Work from home |
| 14:00 (Work from home) | Data analysis | |
| 15:00 (Work from home) | Team meeting | |
| 17:00 (Work from home) | Prepare worksheet and share the schedule of experiment | |
| 18:00 (Work from home) | End of work | |
All-day office work schedule
| 8:30 (Office work) | Pharmacological experiment | Office work |
| 11:30 (Office work) | Break | |
| 12:30 (Office work) | Pharmacological experiment | |
| 14:30 (Office work) | Data analysis, documentation, etc. | |
| 17:00 (Office work) | Meeting with pharmacology member | |
| 17:30 (Office work) | End of work |
Employee Q&A
 Translational Research/Pharmacokinetic Researcher
Translational Research/Pharmacokinetic ResearcherGraduated from the Department of Integrated Pharmaceutical Sciences, Graduate School of Medicine and Pharmaceutical Sciences
Yosuke Yamanaka
What are your current work roles and responsibilities?
In the optimization research of drug candidates, we are responsible for refining the candidates from a pharmacokinetic prospective.
Pharmacokinetic research evaluates how a drug is absorbed, distributed, metabolized, and excreted in the body. While pharmacology and toxicology research evaluate the effect of a drug on the body, pharmacokinetic research evaluates the effect of a body on the drug in a time-dependent ad quantitiative manner. Based on drug concentrations in target tissues, the concept of drug efficacy is supported and appropriate dosages and dose regimens are determined. Drug metabolizing enzyme identification and inhibition data are also used to predict the risk of drug-drug interactions in humans. Based on the metabolite profiling, we contribute to the selection of appropriate animal species in toxicology studies and to the reduction of the risk of adverse effects in humans. Furthermore, we evaluate the relationship between pharmacokinetics (PK) and pharmacodynamics (PD) using data on biomarkers that support drug efficacy. We also incorporate advanced methods such as Quantitative Systems Pharmacology (QSP) and Microphysiological Systems (MPS) to improve the translatability from animals to humans. We are also working to improve the accuracy of our predictions. Through these evaluations, we contribute to the selection of development candidates that minimize adverse effects in humans and maximize drug efficacy. Selected development candidates are then advanced to the Translational Research & Studies for IND/NDA stage, where their profiles are futher investigated.
What are challenging, interesting, or difficult aspects of your role?
In the case of conventional small molecules, methodologies for pharmacokinetic evaluation have been established to some extent, and we are able to understand pharmacological actions and side effects from the obtained data and to predict them in humans. Recently, the drug modalities have been diversifying, including antibodies, viruses, cells, and nucleic acids. Conventional methodologies are not applicable to these new modalities, and there is a lack of knowledge and know-how in the pharmaceutical industry. In these modalities, pharmacology and toxicology data are often important for understanding pharmacokinetics in addition to the target biology . The team is highly motivated to solve each problem together, and we are able to resolve issues through repeated discussions. This is a role that gives me great satisfaction, where we can establish strategies from scratch in a field in which we have no experience and overcome unknown challenges as a team. Why don't you join us and tackle new challenges?
Yosuke Yamanaka's daily schedule
| 8:00 | Global meeting (discussion about study data, development the strategy, etc.) |
| 9:00 | Commuting |
| 10:00 | Arrive at office and prepare for work (for free-adress office) Check email and respond |
| 11:00 | Internal meeting |
| 12:00 | Lunch |
| 13:00 | Internal meeting |
| 14:00 | Confirm the results, analysis, discussion, and document preparation |
| 15:00 | Meeting with persons in charge of outsourced studies |
| 15:30 | Confirm the results, analysis, discussion, and document preparation |
| 17:00 | (in case of working from home) Pick up children from school childcare |
| 17:30 | Planning of studies |
| 19:00 | Clean up the desk, end of the workday |
Employee Q&A
 Medicinal Chemist
Medicinal ChemistXue Yunwei:Graduated from the Department of Pharmaceutical Sciences, Graduate School of Pharmaceutical Sciences
Satomi Imaide:Graduated from the Graduate School of Pharmaceutical Sciences, Faculty of Pharmacy
What are your current work roles and responsibilities?
Xue:
As medicinal chemists, my job is to drive the design, synthesis, evaluation, and analysis (DMTA; Design-Make-Test-Analysis) cycle of new drug candidate molecules. This stage is at the core of new drug discovery and plays a very important role.
Based on the target information and initial data obtained in the Concept Ideation stage, the DMTA cycle is executed, and finally the optimized development candidate is handed over to the Translational Research & Studies for IND/NDA stage. We may be responsible for multiple themes simultaneously while conducting parallel synthesis. Improving the efficiency of this stage is a challenge for the entire drug discovery process.
In recent years, AI and robotics technologies have made significant progress. Our team has built a platform that utilizes these technologies to shorten the duration of this stage. Specifically, we are utilizing a wide range of functions, including AI-based compound structure design and generation, optimization of reaction conditions, and prediction of physicochemical and pharmacokinetic properties. The introduction of a robotic synthesizer linked to AI not only enables efficient progression of numerous reactions, but also enables structure-activity relationship (SAR) analysis based on acquired data and comparative analysis with AI predictions. Through these efforts, we aim to shorten the time required to acquire development candidates.
Imaide:
Likewise, as a medicinal chemist, I play a manufacturing role in the creation of new development candidates. I am in charge of setting up the appropriate modality that matches the drug discovery concept, starting from the biology that is highly relevant to the disease. In the Engineered Small Molecules (ESM) department, we focus on degradation inducers. We examine the method of acquiring compounds as a starting point, identify the acquired compounds, and then optimize the molecular structure. When optimizing compounds, it is important to utilize structural biology and understand parameters that are highly relevant to drug efficacy and safety. In recent years, we have also been working on the application of antibody-drug conjugates, which requires a high level of technical expertise to create compounds with more complex molecular structures.
The creation of development candidates with guaranteed physical properties, kinetics, and safety is one of the milestones for advancing to the next stage. In addition, research for the supply of compounds for preclinical studies and the manufacture of compounds for clinical studies can be considered from the early stage when development candidates have been narrowed down, leading to the formulation of a more reliable strategy.
In addition, for validation of a drug discovery concept, we may provide compounds that contribute to the validation. We will discuss how to prove the concept based on the findings obtained in the course of the evaluation and promote the project.
What are challenging, interesting, or difficult aspects of your role?
Xue:
The most rewarding part of this stage is the moment when a compound that we have designed and synthesized shows efficacy in in vitro and in vivo experiments, and we see its potential to ultimately contribute to patient treatment. As a medicinal chemist, I feel a great sense of fulfillment when the molecular structure on the blueprint is transformed into a development candidate through efficient synthesis, evaluation, and analysis using chemical methods integrated with AI and other technologies. Another attractive point is that I am exposed to new knowledge and ideas on a daily basis at the forefront of pharmaceutical research.
On the other hand, I also face many challenges at this stage. For example, the correlation between the activity and structure of a designed molecule often does not show the expected results, and to determine the cause of this, a depth of expertise and flexible thinking are required. In particular, in recent years, the diversification of modalities has made AI predictions difficult to apply in some cases, and improving prediction accuracy requires a continuous supply of appropriate data to the model. Furthermore, multiple research themes (some from overseas!) in parallel, efficient schedule management and smooth communication among the team are essential.
Imaide:
I find it rewarding to be able to design with my own hands what could become a development candidate or even a pharmaceutical product. I work with the image that the compounds I am involved in research will be delivered to patients as pharmaceutical products and contribute to their health and treatment.
In my research, I enjoy the scientific process of finding the seeds of a compound and brushing up the compound. When I make a scientific discovery that is unknown to the world, it is as interesting as solving a mystery. I also enjoy working as one Astellas, a team of professionals working together toward the goal of pharmaceuticals, and the interaction with colleagues in overseas offices provides me with opportunities to gain new perspectives.
Our current challenge is how to control targets that are attractive for the treatment of diseases but difficult to control. Modalities and know-how that can control these targets are in demand. For this reason, we are accumulating knowledge within Astellas and constantly keeping a close eye on global scientific advances as we conduct research. We are constantly striving to meet the challenge of linking scientific advances to pharmaceuticals.
Xue Yunwei's daily schedule
| 8:30 | Arrive at office, check email, organize tasks for the day |
| 9:00 | Molecular design with AI support, develop a new synthesis strategy |
| 10:00 | Compound synthesis experiments (hand synthesis/parallel synthesis/robotic synthesis as appropriate) |
| 12:00 | Lunch break |
| 13:00 | Weekly meeting / discussion or inter-departmental collaboration meeting (progress report, health and safety check, information sharing and review of next synthesis strategy) (progress report, health and safety, information sharing, and consideration of next synthesis policy) |
| 14:00 | Check results of physical properties analysis of synthesized compounds (LC-MS, NMR, etc.) |
| 14:30 | Check results of synthesis experiments, post-processing, purification, and analysis requests |
| 16:30 | Provide activity data of compounds to AI for analysis and reflect them in the next molecular design Reflect |
| 17:30 | Prepare for the next day |
| 18:00 | End of the workday |

Translational Research & Studies or IND/NDA
It's a research stage to explore the indicators of clinical efficacy of development candidates and to confirm their safety in humans.
The obtained development candidates are examined in more detail in terms of their profiles, including efficacy, safety, and pharmacokinetics in the body. We also conduct studies necessary to develop strategies for clinical trials and drug approval applications, to fulfill regulatory requirements, and to contribute to sales promotion after obtaining approval.
Employee Q&A
 Non-clinical Safety Researcher
Non-clinical Safety ResearcherGraduated from the Department of Natural Resources and Biological Sciences, Faculty of Agriculture
Daisuke Kigami
What are your current work roles and responsibilities?
I am responsible for providing information from a safety perspective in the selection of development candidates to be advanced to clinical trials.
And even after clinical trials have been started, safety research is continued, and I provide the necessary information at each stage from phase 1 to phase 3 and up to the application for approval of a new drug.
Specifically, before initiation of clinical trials, we conduct safety studies of development candidates in accordance with drug guidelines, and the obtained information leads to a risk management plan for side effects in the clinic.
Until an application for approval submission, it is necessary to clarify the value of the new drug for patients, and it is important to demonstrate the risk-benefit of efficacy and safety. Safety research contributes to clarify this risk.
On the other hand, the role of safety research in translational research is broad and includes early drug discovery studies. We evaluate the safety of on-target and off-target not only in vitro and in vivo, but also in silico and from multiple perspectives, thereby contributing to the selection of higher quality drug candidates.
What are challenging, interesting, or difficult aspects of your role?
We are developing areas where there are no curative drugs and challenging research such as gene therapy and cellular medicine. We are also evaluating safety research from a molecular biological perspective, such as gene expression analysis, and I spend my days actively engaged in this kind of new and exciting research. Once a development candidate advances to the cinical phase, collaboration with clinical development members in the U.S. and Europe becomes active, and research and development is conducted globally. Because of this global development, there are opportunities to go on overseas business trips to meet with local members and I enjoyed cross-cultural communication.
The most important thing in drug development is, above all, to deliver a new drug to patients who are waiting for that therapeutic drug. I find it rewarding to work hard in research based on the belief that our research activities can meet the needs of patients.
We welcome you to join us in conducting such cutting-edge drug discovery research and creating new drugs with us! We look forward to meeting you all.
Employee Q&A
 Translational Research/Pharmacokinetic Researcher
Translational Research/Pharmacokinetic ResearcherGraduated from the Department of Veterinary Medicine, Faculty of Animal Science
Hiroyuki Sayama
What are your current work roles and responsibilities?
Translational research (TR) is a series of studies aimed at bridging non-clinical research findings to clinical application, encompassing a wide range of activities. We approach TR with the objective of increasing the probability of success of Proof of Concept in clinical development and conduct our research accordingly.
Examples of our TR include the identification of biomarkers to confirm the efficacy of drug candidates in clinical trials and the evaluation of drug efficacy and/or safety using human tissue-derived samples. The mathematical modeling work I am responsible of (please refer to the linked resource) is also an integral part of our TR initiatives. During the concept validation stage, hypotheses for the mechanism of the action of a drug candidate is examined through various experiments. By analyzing these experimental data using mathematical models, we aim to deepen our quantitative understanding. Furthermore, by incorporating species differences between animals and humans into the mathematical models, we are able to predict candidate profiles in humans and contribute to the design of clinical trials. In essence, mathematical modeling is a core TR activity that translates diverse preclinical findings into a quantitative framework and predict clinical outcomes, serving as a true bridge between non-clinical and clinical.
What are challenging, interesting, or difficult aspects of your role?
The application of mathematical models in drug development has a long history. Traditional models, such as pharmacokinetic models, which describe the disposition of drug candidates in the body, and pharmacokinetic-pharmacodynamic models, which capture the relationship between drug exposure and pharmacological effects, have been widely used. In recent years, a new type of mathematical model known as the Quantitative Systems Pharmacology (QSP) model has gained increased attention, and we are actively focusing our effort in this area. QSP models aim to represent the underlying biological mechanisms of disease, allowing us to analyze complex interactions between drug actions and physiological responses. Therefore, QSP modeling requires not only advanced model-building skills, but also a deep understanding of biology. Although this is a very challenging task, I find it highly rewarding to collaborate with pharmacologists in developing these models, and it is an exciting moment the model successfully reproduce the data. These QSP models, once developed, are used for various clinical purposes. Recently, we have been particularly focusing on supporting clinical trial planning by predicting the pharmacological effects of combination therapies involving of our investigational drugs and marketed drugs. Being able to contribute directly to actual clinical development through such modeling work is both interesting and deeply fulfilling.
Employee Q&A
 Pharmacology Researcher
Pharmacology ResearcherGraduated from the Department of Pharmacy, Faculty of Pharmacy
Mako Numazaki
What are your current work roles and responsibilities?
I have two main responsibilities. First is to formulate the stories and strategies for the pharmacological studies required for investigational new drug application (IND) and new drug applications (NDA). Second is to ensure the reliability of these preclinical pharmacological studies.
The development candidates selected during the Candidate Creation & Optimization stage in each project, are subsequently subjected to clinical trials, where they are administered to volunteers and patients to evaluate their safety and efficacy. To initiate clinical trials, an IND must be submitted to the regulatory authorities in each country with accurate non-clinical data. After long termed clinical trials, an NDA must be filed for a development product that has demonstrated promising results in clinical trials to bring it to market.
In the Translational Research & Studies or IND/NDA stage, we manage to develop the pharmacological study story and strategy, and to prepare a reliable pharmacological study report required for submission in collaboration with the researchers involved in the Candidate Creation & Optimization stage. Our collaboration extends not only internally but also with external resources such as contract research organizations (CROs) and collaborative research to prepare a pharmacological study report. In some cases, we also conduct research to support post-launch product promotion.
In addition, it is crucial to ensure the reliability of non-clinical pharmacological studies, we optimize operational systems and rules to guarantee with reliability, and we provide consultation on all aspects of non-clinical pharmacological studies from study design to report preparation. This includes collaboration with the Quality Assurance department and providing reliability education for researchers.
What are challenging, interesting, or difficult aspects of your role?
Our work to lead up to a drug approval application is the most exciting and challenging. During this period when the countdown begins for a new drug to reaches patients, accuracy and speed are required in our daily work, we deeply consider that each of our work is directly related to the light side for patients, which makes the work both responsible and rewarding. Filing for approval and dealing with the regulatory authorities are responsibilities that are special to a pharmaceutical company, and the ability to learn different perspectives from those of research is also a major attraction.
Another appealing aspect of this role is the many opportunities to broaden your knowledge by being involved in a variety of projects, both in Japan and overseas, which increases your exposure to cutting-edge science. It is very rewarding to be able to bring our expertise together and work as a team to propose drugs to the world that do not yet exist.
In non-clinical pharmacological study, the most important thing is to ensure reliability. Our policy is not only to comply with existing rules but also to optimize operational systems and rules to ensure reliability. So, exchanging opinions with members of research centers around the world is a valuable opportunity for us to learn from each other's perspectives. Exchanging views with pharmaceutical companies is also a valuable opportunity.

Lessons learned for new concept ideation
When the efficacy and safety are revealed in clinical trials, and the regulatory authorities recognize the value to patients, the drug will be launched. In some cases, the insights gained from clinical trials can also lead to new ideas for drug discovery. By continuing this cycle, pharmaceutical companies are able to continuously provide value to patients. We will also conduct research to deepen our understanding of the correlation between outcomes in non-clinial studies and clinical trials.
Work Location
-
Tsukuba Research Center (Ibaraki, Japan)
Access Map






