
INTRODUCTION OF
THE PHARMACEUTICAL
TECHNOLOGY DEPARTMENT
Job Description
The pharmaceutical technology research professionals conduct technological research related to
discovering the new candidates with high developablity, drug substance manufacturing processes and
formulation, industrialization research that takes into consideration the environment, quality, and
safety, supply investigational products necessary for the start of clinical studies based on development
strategies, prepare materials for approval application through cooperation with quality assurance and
respond to factory inspections, develop mechanisms to supply products in line with sales strategies, and
provide technical guidance for the transfer of manufacturing technologies to overseas hubs, and facility
design. They play a key role in which Astellas speeds up the launch and supply of high-quality new
products. The engineering professionals are responsible for introducing research equipment, building
investigational product manufacturing facilities based on pharmaceutical technology research, and
building manufacturing plants for future commercial production.
Pharmaceutical technology research professionals conduct commercial and CTM production of chemical drug
substances, solid dosage forms, sterile injectables, fermentations, antibodies, and biopharmaceuticals
under strict GMP controls. Pharmaceutical technology research processionals also conduct facility
suitable manufacturing process development, pilot scale process verification, and further process
modifications utilizing the pilot results. By supplying pharmaceuticals globally, we have gained
experience in testing both domestically and internationally, and are building a higher-level quality
system to ensure a stable supply of products to patients.

-
Chemical & Biological Technology Labs. (CBTL)
CBTL is responsible for the “Drug Substance” manufacturing process.
VIEW MORE
The modalities covered by the CBTL are quite diverse, from chemically synthesized products to fermentation products, biologics, virus drugs, and cell therapy products derived from stem cells. In addition, we have introduced cutting-edge robotics and digital technologies, and are diligently proceeding with research into continuous manufacturing technology for antibody and the mechanization and automation of DS manufacturing processes and operations for cell therapy products. -
Analytical Research Laboratories
In order to deliver drugs with high functionality and appropriate quality to patients, the Analytical Research Laboratories supports Astellas’ development of manufacturing technologies from the quality aspect through high-level product understanding and analytical development based on physicochemical, biochemical, and analytical chemistry from the late stages of pharmaceutical product development after the acquisition of proof-of-concept (POC) to post-marketing.
VIEW MORE -
Pharmaceutical Research and Technology Labs
The mission of the Pharmaceutical Research and Technology Labs is to deliver highly functional, high-quality new drug products to patients and medical settings around the world in a timely manner.
VIEW MORE -
CMC Research
Pharmaceutical Science & Technology Labs (PSTL) is in charge of a broad range of pharmaceutical technology research operations for all early-stage development projects in all modalities, such as small molecules, antibodies, proteins, nucleic acids, viruses, cells, and the new challenge of Rx+ products, etc. We strive to obtain “the fastest proof-of-concept (POC)” for development-stage products by leveraging our strong manufacturing/analytical technological capabilities.
VIEW MORE -
Engineering Group
The Engineering Group works on "engineering" and "technology development" activities. In both of these tasks, we work to optimize the entire procedure based on environmental, safety, quality, delivery time, and cost perspectives. But technology development focuses on installation of new technology, digital technology and automation technology.
VIEW MORE -
Takahagi Technology Center
Takahagi technology center are always challenging to synthesize wide variety of chemicals toward drug substance supply for the commercial and clinical purposes as a leading drug substance manufacturing site in Astellas.
Introduction Working employees Working place -
Yaizu Technology Center
As the lead plant for drug products and biopharmaceuticals, we are strengthening our coordination with the Formulation Research Center to enhance our commercialization technologies and expedite stable production.
Introduction Working employees Working place -
Toyama Technology Center
Toyama Technology Center is the base for the biological commercial products of Astellas. In addition to the manufacturing of commercial products, we also improve the manufacturing processes, scale-up the process in the late development stage, and manufacture the active pharmaceutical ingredients for the clinical study use. Through these activities, we also play an important role in development of engineers who will be responsible for stable product supply into the future.
Introduction Working employees Working place -
Takaoka Plant
The Takaoka Plant specializes in manufacturing of injection products.
Introduction Working employees Working place
We have several capability to manufacture injection products and continue to evolve in line with the times as a base for the development of injection products.
Chemical & Biological Technology Labs. (CBTL)
CBTL is responsible for the “Drug Substance” manufacturing process.
The modalities covered by the CBTL are quite diverse, from chemically synthesized products to
fermentation products, biologics, virus drugs, and cell therapy products derived from stem cells. In
addition, we have introduced cutting-edge robotics and digital technologies, and are diligently
proceeding with research into continuous manufacturing technology for antibody and the mechanization and
automation of DS manufacturing processes and operations for cell therapy products.
Process Chemistry
We have achieved a "stable supply of drug substances" using chemical synthesis
technology. Our main activities are as follows; (1) developing manufacturing methods for synthetic
drug substances, (2) transferring technology to production sites, manufacturing and supplying large
amounts of drug substances, (3) life cycle management of pharmaceuticals (manufacturing troubles,
stockout avoidance, cost reduction, etc.). In order to ensure a stable supply of drug substances, we
are developing cost effective manufacturing method to deliver high quality drug substances,
considering safety and the environment. Therefore, we are a group of specialists with not only organic
synthetic technology but also multiple technology and knowledge such as separation and purification,
analysis, production, chemical engineering, safety environment, GMP, and regulatory requirements. In
addition, we are also devoted to acquiring new technologies such as flow synthesis, fine bubbles, and
experimental research automation, etc.
We have the only EHS research function in Astellas. As for safety measures, we evaluate all synthetic
processes before manufacturing, and as for environmental measures, we evaluate activated sludge
process at inside and outside production sites.
We also provide technical supports at overseas production sites and apply for new drug approvals to
overseas authorities such as the FDA and EMA. Therefore, there are opportunities for researchers to
play an active role globally, not just in examining experiments within the laboratory.
Bioprocesses Science
Bioprocess Science is responsible for process development for manufacturing
biological drug substances.
mAb an example, the bioprocess can be divided into cell line development,
cell culture process development and purification process development.
Cell line development
The construction of antibody-producing cell line is like a treasure hunt. It is a
task to create and find robust CHO cell line that have high antibody productivity, appropriate product
quality, and can withstand large-scale cell culture in future commercial production. We have been
building a platform how to integrate antibody genes into CHO cells and how to find better candidates
efficiently by accumulating experience.
There is the pressure of tight deadlines, however, we sincerely collect and analyze data for thousands
of candidate cells and select right one that will support subsequent production. We believe this bring
VALUE to patient.
Cell culture process development (Uptream Bioporcess Science)
We are mainly in charge of the cell culture process development for producing
macromolecules such as antibodies using CHO and microorganisms. We cover a wide range of product
lifecycle, from supplying research sample at the initial development stage to establishment the
process for stable production, technology transfer to manufacturing sites, biologics license
application, and supporting the manufacturing of commercial products.
The role of cell culture process development is to establish a stable culture process even on a large
scale. In order to stably deliver drugs to patients, it is essential that the manufacturing process is
highly reproducible and operates stably. We are deepening our understanding of the effects of process
parameters such as temperature, pH, etc. on cell culture performance and product quality, and we are
establishing robust culture processes with scientific knowledge. In addition, we improve productivity
by optimizing culture medium and culture conditions, improve quality, reduce costs, and troubleshoot
when problems occur in manufacturing.
Purification process development (Downstream Bioprocess Science)
We are responsible for purification process development for biologics. There are many impurities and target substance-derived impurities/related substances in the cell culture solution. In order to efficiently isolate and purify high-quality target substances from culture fluids, we establish a low-cost and environmentally friendly purification process by combining the cutting-edge technologies such as solid-liquid separation, column chromatography, and membrane concentration. For industrialization, we are also working on process automation research to improve the efficiency of manufacturing processes.
Process Engineering
There is a process engineering laboratory that fulfills gaps between process development and commercial production with bio-& chemo-engineering, mechanization/automation, and Dx technology such as simulation.
Main research themes
[Process Chemistry]
Process development of synthesis routes for new drug substances with pharmacological activity and
technical support during manufacturing
Manufacturing process development assuming commercial production and technology transfer to
manufacturing site
Environmental and health safety research related to the entire drug substance manufacturing process
Approval applications worldwide including Japan, Europe, and America, and technology transfer to
manufacturing sites
Cutting edge technology development
[Bioprocesses Science]
Process development for manufacturing biological drug substances aiming at world-class standards
Technology transfer of manufacturing process for biological drug substances to manufacturing sites
Biologics license application and supporting the manufacturing of commercial products
Cutting edge technology development
A Message from the head of Chemical & Biological Technology Labs.

Hideto Yamaguchi
In April 2020, the Chemical & Biological Technology Labs. was established by merging the Synthetic
Processes Research Laboratory, responsible for the development of synthetic processes for small
molecule drugs, and the Bioprocesses Research Laboratory, responsible for the development of culture
and purification processes for biopharmaceuticals such as antibody products, in order to fully realize
the utilization of CMC research and development for various modalities.
The modalities covered by
the Chemical & Biological Technology Labs. range from chemically synthesized compounds to fermentation
products, biopharmaceutical products, and even virus-based products and cell-based products produced
by inducing differentiation of stem cells. If it can become a pharmaceutical product, the Chemical &
Biological Technology Labs. is in charge of the entire development of the drug substance manufacturing
process. In addition, for modalities with immature drug substance manufacturing technologies such as
viruses and cells, we introduce state-of-the-art robotic and digitalization technologies, and we are
advancing research on the mechanization and automation of drug substance manufacturing facilities and
operations.
It takes 10 years to get a drug on the market. During this period, the manufacturing process
development begins with the acceptance of the manufacturing method in the early stage of research, and
it requires a deep scientific understanding of the target product and manufacturing process,
development of a robust process that can withstand commercial use, acquisition of regulatory approvals
in each country, and technology transfer to domestic and overseas manufacturing plants. Even in the
same process development, there are different challenges in each development phase, and researchers
affiliated with the Chemical & Biological Technology Labs. have the opportunity to accumulate a lot of
experience in pharmaceutical product manufacturing.
Global development is one of the characteristics of Astellas’drug substance process development
research. Under Astellas’ vision of "On the forefront of healthcare change to turn innovative science
into VALUE for patients," we continues to add companies and researchers who are at the cutting edge of
science overseas to our organization. By combining our previous experience in manufacturing with
advanced science, we are taking on challenges every day to bring first-in-class and unique
pharmaceutical products to patients as quickly as possible.
Astellas is looking for passionate
researchers who can work globally while valuing teamwork, and who can personally create their own
future and innovative research areas.
Employee Q&A

I study the method for the scale-up synthesis of drug substances, which is active pharmaceutical ingredients.
Master of Molecular Pharmaceutical Science, Graduate School of Pharmaceutical
Sciences
Joined the company in 2022
Honoka Teraji
-
What is your current job description?
I study the method for the scale-up synthesis of drug substances, which is active pharmaceutical ingredients.
In order to deliver pharmaceuticals to patients around the world, we must be able to scale-up synthesis of drug substances. Since the quality of pharmaceuticals directly affects the health of patients, a manufacturing method that can stably supply high-quality drug substances is necessary. In scale-up synthesis, it is also necessary to consider the environment and safety, and to aim for cost reduction.
At the Chemical & Biological Technology Labs, we are developing manufacturing methods that enable the stable, safe, and low-cost supply of high-quality drug substances. We are responsible for work related to drug substances in a wide range of development phases, from lab-scale experiments to large scale manufacturing for commercial products and post-marketing life cycle management. In addition, we are able to be involved in operations necessary for the stable supply of drug substances, such as submitting applications to the regulatory authorities and managing starting material sources and manufacturing sites.
I am in charge of small molecule drugs in the early stages of development, and developing manufacturing methods for the first chemical synthesis of drug candidate compounds on a kg scale. The drug substances produced will be used for formulation studies and clinical trials, so it is necessary to establish a manufacturing method that enables synthesis with a predetermined quality and schedule. Within a limited time frame, I search for appropriate routes and reaction conditions by repeating the cycle of hypothesizing, experimenting, and discussing. I also discuss with my colleague to get hints and obtain information through literature research, challenging myself to improve the manufacturing method to a high quality. When I was in university, I often thought only about the "target compound," hoping to synthesize it, but since joining the company, I have been able to think more deeply about "how to synthesize" compounds.
I feel each job is worthwhile when I think that there are patients waiting for the drugs we synthesize. -
When do you find work interesting or difficult?
Synthesizing large quantities of drug substances is difficult, but also interesting and fulfilling work.
I find the process of achieving scale-up synthesis both interesting and difficult. When deciding on a synthesis method in the laboratory, we consider many things. For example, the reaction conditions for high yield, whether the reaction proceeds in the same way each time, how to synthesize from even the cheapest starting materials, how to remove or reduce impurities, how to reduce the workload at the manufacturing site, and so on. There are many items to be considered, and I feel that it is difficult to choose a policy and selection for consideration. That said, I find research interesting when I can prove a hypothesis or find the cause of a problem.
If I were to do chemical synthesis on my own, I would be limited to a few 10g scale at most. I was surprised to see how many people are involved in the safe and high-quality synthesis on the scale of several 10kg to several 100kg. For example, we work with an environmental safety team that confirms that the manufacturing process we have created is safe for operations, a quality assurance department that confirms that the quality of the drug substances can be assurance using the established manufacturing process and test methods, and the manufacturing site where is actually produced according to the manufacturing process. I feel a great sense of accomplishment when I succeed in synthesizing a large amount of drug substances as a result of our close collaboration with the relevant departments. -
In your work, what challenges would you like to take on in the future?
I would like to take on the challenge of working in a wide range of phases, from the early stages to commercial stage.
I would like to become a process chemist with a broad perspective and would like to experience all the processes involved in the supply of drug substances. From synthesizing a small amount of a drug candidate compound to putting it on the market and managing its stable supply, I need to understand not only organic chemistry but also chemical engineering, safety engineering, application-related laws and regulations, and many other fields. Furthermore, in order to work smoothly as a team throughout the company, it is necessary to understand and consider the processes before and after my own department. Since many of the starting material manufacturers and manufacturing plants are overseas, communication in English is also important. In my second year with the company, I am still learning, but I would like to continue to take on challenges in my daily work. I believe that the environment in which I can take on the challenges of projects in various phases is only possible at the Chemical & Biological Technology Labs, which handles everything from initial development products to commercial products in one place.
-
What is the appeal of working at Astellas?
I am attracted to a work environment where I can take on a variety of challenges and work easily.
We feel that this is an attractive environment in which to work. First of all, there is an environment where you can concentrate on your experiments. We have a wide variety of equipment, reagents, and analytical instruments for the experiments we want to conduct. We can work in teams of several people to challenge new trial, and we receive support in various ways, such as the introduction of necessary equipment. In addition, there is a culture of easy communication with people in the workplace. Regardless of the year, we are able to help each other by sharing our opinions and suggestions and receiving advice on experiments. New ideas are often born out of chit-chat. It is a good work environment in terms of work-life balance. FF Day, in which finish work earlier on Fridays, has been introduced, and paid vacations are easy to take, so employees can refresh themselves regularly. Many employees work according to their life events, such as male employees taking maternity leave.
Another major attraction of this company is that employee growth is generously supported. Rotational training, in which employees visit related departments, allows them to deepen their understanding of their work and how their own department's work is related to the processes before and after them. When I participated in training for a department that handles biopharmaceuticals in the same laboratory, it was an opportunity to deepen my understanding of biopharmaceuticals and reconsider the characteristics of small molecule pharmaceuticals. In particular, we have a significant relationship with the manufacturing plant, which synthesizes drug substrates using the manufacturing process we created, allowing us to experience the manufacturing site over a six-month period. The scale-up from the laboratory to the manufacturing site is not simply a difference in size. The perspectives that can be learned from the manufacturing site are also important for future process development, and I find it attractive to be able to experience this as training. In addition, we have other systems in place to support self-improvement, such as participation in domestic and international academic conferences and support for degree acquisition. The fact that there are many people challenging themselves to achieve their goals is a good stimulus, and I think it is an environment where we can grow together.
A day in the life of Honoka Teraji
| 8:30 | Arrive at office, check email and daily schedule |
| 8:30 to 12:00 | Experiment : Prepare and track reactions, collect experiments from the previous day |
| 12:00 to 13:00 | Lunch at the company cafeteria. You can have a delicious lunch with many varieties at a reasonable price. |
| 13:00 to 15:00 | Sometimes there are meetings with the team or research contractors. |
| 15:00 to 15:30 | Take a break for discussion at the company café. There is a daily menu, which is fun. |
| 15:30 to 18:00 | Summary and discussion of experiments and confirmation of tomorrow's schedule. |
| 18:00 | Leave the office. Sometimes we go out to dinner with our peers. |
Employee Q&A

Development of the cell culture process for antibody pharmaceuticals.
Completed Ph.D. in Computational Biology and Medical Sciences
Joined the
Company in 2021.
Masaru Shimojo
-
What is your current job description?
Development of the Cell Culture Process for Antibody Pharmaceuticals.
I am working on the development of cell culture processes for the production of antibody pharmaceuticals. Pharmaceutical companies have a responsibility to consistently deliver high-quality and safe products to the many patients in need. To achieve this, it is necessary to develop a process that not only verifies the effectiveness and safety in clinical trials but also produces and supplies high-quality products. In the case of antibody pharmaceuticals, it is necessary to develop a cell culture process that maximizes the productivity of the cell line producing the antibodies and demonstrates stable quality.
Furthermore, the developed process must not only be demonstrable at the laboratory scale but also ensure equivalent performance when scaled up to bioreactors hundreds to thousands of times larger than the laboratory scale. Therefore, we are concurrently working on various technological developments in parallel with our process development tasks, utilizing them to enhance the development of better processes and their scale-up evaluations.
In addition, process development work is sometimes carried out in collaboration with other companies. We discuss the planning of experiments and interpretation of results needed for process development while progressing together. As we often collaborate with overseas companies, there are opportunities to engage in global work, which requires proficiency in English for effective communication. -
When do you find work interesting or difficult?
The Fascination of Encountering "New Things" and the Challenge of Solving Them
What I find interesting about this job is that I encounter a lot of "new things." In the field of biopharmaceuticals, there are many "new things" such as the process development for new antibody molecules or New Modalities, advancements in technology, the introduction of new equipment, and the absorption of new knowledge. However, for every encounter with something "new things," an equal number of "challenges" arise. "Challenges" like developing the suitable processes for new molecules/New Modalities and establishing the operational systems to ensure that newly introduced equipment is accessible to everyone, and more, also emerge in abundance, and more, also emerge in abundance. It can be difficult to solve these "challenges" on my own, but through discussions and verifications with the team, we can lead to new discoveries and find solutions, which in turn adds to the excitement. I consider myself fortunate to be able to work at Astellas, where such an environment exists, as I have a curious and inquisitive nature.
-
In your work, what challenges would you like to take on in the future?
Reliable and Swift Process Development and Challenging Technological Advancements
In pharmaceutical process development, I understand that ensuring reliability in the work is crucial, as it is necessary to develop a process for producing active pharmaceutical ingredients stably without causing delays to the schedule. In recent years, Astellas has seen an increase in achievements in process development for antibody pharmaceuticals, and a platform for process development is beginning to take shape. However, many specialized antibodies like bispecific antibodies come into play on the development stage. It becomes necessary to optimize the process for each molecule. Therefore, I aim to leverage scientific knowledge and experience for any type of molecule, in order to swiftly develop a reliable process.
Additionally, new technologies in the field of biopharmaceuticals are advancing at an astounding pace. To avoid falling behind in the industry, it's crucial to constantly monitor new technologies, evaluate their potential usefulness for our company early on, and take in those valuable technologies. While implementing such technologies may take time, I consider it a highly rewarding endeavor, as it could potentially elevate our company's technological capabilities. I'm eager to take on these challenges actively. -
What is the appeal of working at Astellas?
Open and Transparent Work Culture
Even as a young professional, I feel comfortable expressing my opinions in this open and communicative work environment. As a result, each individual is able to showcase their unique personalities and expertise while collaborating effectively on tasks. In meetings, there is active exchange of ideas, regardless of whether someone is a young professional or a seasoned veteran.
Furthermore, I perceive that young professionals are provided with numerous opportunities for growth, such as participation in conferences. While the shift to online participation has increased due to the impact of COVID-19, there are still opportunities to attend international conferences locally, allowing us to stay updated with the cutting-edge developments in the industry. If there is an interest in new technologies from other companies, regardless of one's seniority, arranging meetings or attending seminars is possible, facilitating information gathering.
Moreover, the company as a whole is dedicated to promoting work-life balance, including both work and parenting. In my department, it is relatively easy to take maternity or paternity leave, and many individuals, regardless of gender, have taken advantage of this opportunity. I myself took paternity leave when my first child was born.
A day in the life of Masaru Shimojo
| 6:00 | Wake up. Get ready with the family. |
| 7:30 to 8:30 | Commute to work. Review the day's schedule. |
| 8:30 to 9:15 | Daily team meeting. Review progress and coordinate tasks. |
| 9:15 to 12:00 | Conduct experiments. Collaborate with the team. |
| 12:00 to 13:00 | Lunch in the company cafeteria. Take a break to prepare for the afternoon. |
| 13:00 to 16:00 | Meetings. Share experiment results and engage in discussions as needed. |
| 16:00 to 17:45 | Desk work. Plan experiments, prepare reports, conduct literature research, etc. |
| 17:45 | Leave work. Pick up my daughter from daycare, and return home. |
Pharmaceutical Research and Technology Labs
Overview
The mission of the Pharmaceutical Research and Technology Labs is to deliver highly functional,
high-quality new drug products to patients and medical settings around the world in a timely manner. Up
until now, we have created outstanding pharmaceutical technologies, such as solubilization of poorly
water-soluble drugs, controlled-release of drugs, and drug delivery systems (DDS) to create additional
value in products, all of which can provide value to patients. We contribute to projects at a wide range
of stages, from the creation of new themes with Research teams, through pre-clinical and clinical
development stages, applications for new drug approval, and technology transfer to commercial
manufacturing sites, as well as the stable supply of drug products. In other words, contributions of
pharmaceutical research starts at the very early research stage to evaluate the physicochemical and
biopharmaceutical properties of drugs, proposal and investigation of DDS concepts, and continues until
commercialization, including formulation development, process development, scaling up for
industrialization, and packaging and device development. For example, we are constantly collaborating
with production facilities in Japan and overseas (US, Europe, etc.) through various activities such as
technology transfer of new products, process improvement, cost reduction of existing products, and
strive to establish manufacturing know-how that is excellent in cost performance and environmentally
friendly.
In addition to conventional small molecule compounds and antibody drugs, we have recently been working
on formulation research targeting new therapeutic modalities such as nucleic acid drugs, and cell
medicines, and are promoting formulation development research globally in collaboration with overseas
development bases (Chicago and Boston in the US, the Netherlands in EU, etc.) as well as domestic sites
(Yaizu and Tsukuba).
Furthermore, we have developed novel drug products with enhanced functionality for key products,
contributing to the maximization of value for these products. If pharmaceutical products are used in
combination with superior pharmaceutical technology including DDS, the value of these products will
continue to grow. Recently, we have been actively working on apps development, formulation design AI and
Dx. We will continue to advance Astellas' pharmaceutical technology as much as possible, nurture
products supported by our technical capabilities, and provide them to the world.
Relation between drug development and Pharmaceutical Research and Technology Laboratories
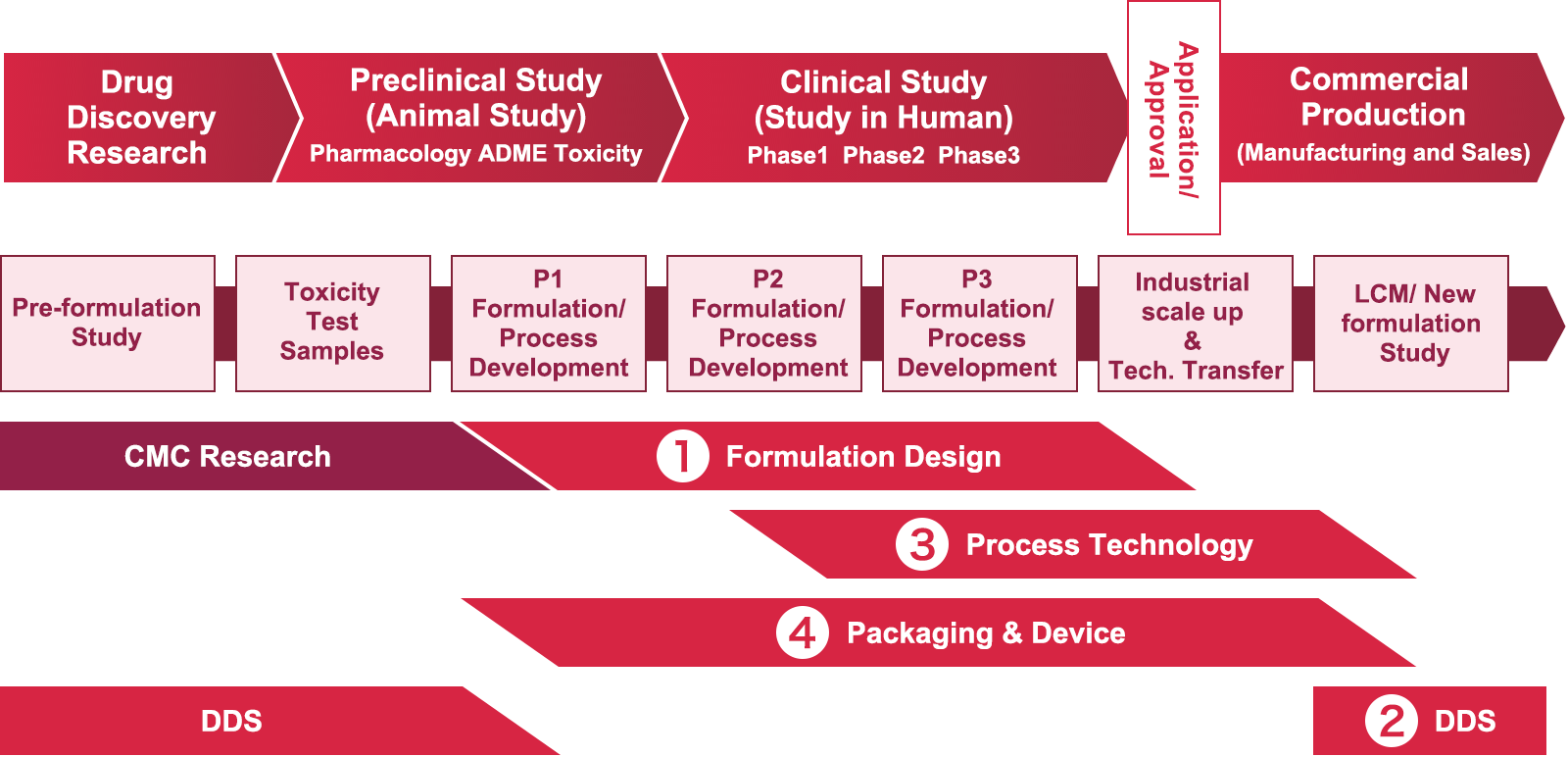
In order to demonstrate the efficacy and safety required for drug products, any one unit (= 1 tablet, 1 capsule, 1 vial) has the same quality, and excipients are necessary to make it easy for patients to use. The main task of the formulation design is to determine the optimal combination and optimal amounts of excipients to be added to the formulation (= determination of formulation), and to design the formulation to the optimal dosage form. It is a heavy responsibility, but it is a very rewarding job, because formulation has a big impact on whether a drug product is easy to manufacture, easy to use, and highly safe and effective.
Since the role required for the formulation differs depending on the clinical phase, we will refine the formulation by identifying the characteristics of the active ingredients while developing formulation that satisfies the goals of each phase. Usually, the final formulation (= the formulation to be marketed in the future) is decided by the late-stage clinical trial and supplied as clinical trial materials. In recent years, development of formulation design AI is being pursued in order to achieve fast and efficient formulation development.
On top of that, while proceeding with the formulation design activities, we will investigate the manufacturing method and manufacturing conditions, as well as the packaging. Therefore, the information obtained from these investigations will be the basic information for commercialization (mass production) activities after the late-stage clinical phase at the Process Technology and packaging design activities.
Drug Delivery System (DDS) is the formulation technology to control distribution of drug substances in the body after dosing. To improve efficacy, adverse effects and user-friendliness, DDS lab. is researching technologies to deliver drug substances (small molecules, antibodies, proteins, nucleic acids, cells, phages etc) to target sites more efficiently and certainly.
For the purpose of "delivering DDS products of values to patients", we investigate, propose, prepare and evaluate (in vitro/vivo) many kinds of formulations such as nanoparticles, gels, local formulations, injectables, oral dosage forms for various target sites and diseases of the below (1) and (2). (1) For needs of drug discovery, we study DDS technologies with researchers of several fields, find the optimal formulations for clinical studies, and apply patents. (2) For drug substances after launch or in late clinical stages, we study new formulation idea to enhance values with members of development and sales.
Our labs are in Tsukuba Ibaraki and Yaizu Shizuoka. We’re collaborating with formulation design researchers and CMC-Research for CTM supply, and package and device researchers for device application. We're also developing new DDS technologies for cell therapy with AIRM in US.
The main task of the Process Technology is developing a manufacturing process for commercial production with high quality, high efficiency and low cost by using final Drug product formulation developed by the Formulation Design.
Participating mainly from late clinical phase, we will lead a technology transfer to global manufacturing sites collaborating with many related departments inside and outside the company. Especially, the new aseptic drug product manufacturing lines are newly established in Yaizu for the increasing importance of aseptic products. We contribute enhancing our aseptic manufacturing capability through the process development.
In new modalities such as cell therapy products, which have made remarkable progress in recent years, we initiate an investigation from early stage because it is extremely difficult to establish manufacturing reproducibility due to their quality characteristics.
The development of new technology is also an important mission, and we are contributing to stable production of new products through the development of cutting-edge data mining systems for the objective of utilizing big data in pharmaceutical plants and research on Process Analytical Technology.
With the motto "Research that is close to patients around the world", we are working on the design and development of packaging and devices that correspond to the diversifying modalities of pharmaceuticals.
We design packaging based on the selection of appropriate materials and maintain the quality of the product over time. We contribute to the reduction of the environmental impacts of our products through the development of environmentally friendly packaging technology, such as the world's first successful use of biomass-derived plastic in blister packages as the primary packaging for pharmaceutical products. Since packaging and devices are touchpoints with patients and healthcare professionals, it is important to ensure not only medical safety through measures such as prevention of medical malpractice and countermeasures against counterfeit drugs but also medication adherence and quality of life through the application of universal design.
In recent years, we have also developed combination products that combine pharmaceuticals and medical devices, digital apps (Software as Medical Device) development for DTx (Digital Therapeutics) , and new technologies such as packaging automation with collaborative robots.
Main research themes
Formulation development/process development, industrialization, and package development for new drug
products
Formulation development/process development, industrialization, and packaging development to maximize
the value of existing products
Study on Drug Delivery Systems (DDS) to improve efficacy and safety
Development of new dosage forms with high functionality or convenience, new formulation technologies,
combination products with pharmaceutical products and medical devices (devices), and new packaging
forms
Formulation development of various therapeutic modalities such as small molecules, antibodies, nucleic
acids, and cells.
Improvement of oral absorption of poorly soluble drugs
Research on process analytical technology to understand and control the critical quality attributes that
affect product quality
Implementation of big-data analyses on commercial production sites for higher product understanding and
proactive process improvement
Scale-up research for various formulations and manufacturing methods
New industrialization research on various formulations and manufacturing methods (process and equipment
development) Study on optimization of manufacturing conditions for process improvement and quality
improvement
A Message from the head of Pharmaceutical Research and Technology Labs

Hiroyuki Kojima
New compounds with pharmacological activity will finally become pharmaceutical products (medicines)
that can be administered to patients through pharmaceutical research. There are a variety of
formulations, including oral formulations such as tablets and capsules, injectables, transdermal, and
inhalation, etc. In addition, there are a lot of technologies that can be incorporated into each
formulation to optimize/maximize treatment effects. For this reason, researchers with knowledge and
technical skills not only in pharmacy but also in physical chemistry, biology, and mechanical
engineering have been working at Pharmaceutical Research and Technology Labs. The products developed
by our researchers via formulation development, process design, and packaging and device development
are clinically used to treat patients worldwide. In addition to small molecules and antibodies, we
have recently been focusing on pharmaceutical research of cell-based and mRNA LNP products, as well as
development of apps, formulation design AI, Dx, drug delivery systems and new pharmaceutical
technologies to maximize the value of drug product.
As one of the research areas closest to patients, clinical, and pharmaceutical products, we are
honored and proud of our pharmaceutical research that generates products in all modalities. In order
to deliver higher-value pharmaceutical products to patients around the world as soon as possible, why
not work with us at the "Pharmaceutical Research and Technology Labs" where we work hard on research
every day in cooperation with not only domestic but also overseas bases such as Chicago/Boston in the
US and Netherland?
Employee Q&A

We research to find the optimal drug delivery system (DDS) for "drug seeds" having pharmacological effects for enhancing the delivering value for patients.
Master of Pharmacy、School of Pharmaceutical Sciences (6-year program)
Joined
the company in 2020
Ayaka Masuda
-
What is your current job description?
We research to find the optimal drug delivery system (DDS) for "drug seeds" having pharmacological effects for enhancing the delivering value for patients.
“Drug seeds” (active ingredients or drug substances) include various modalities such as small molecules, antibodies, nucleic acids, and cells etc. Pharmaceutical research is converting them into “drug products” that can be administered to patients. In the research area, DDS is a technology to control movement of the drug substances in the body after administration. To improve drug efficacy, reduce side effects, and/or improve patients convenience, our challenge is to create drug products for delivery of the optimal amount of drug substances for the appropriate time to the best sites in the body where the drugs perform their efficacy.
I’m working at DDS Laboratory. We investigate and select the optimal DDS technologies for each modality and issues, when it’s difficult to develop the "drug seeds" to drug products due to their insufficient efficacy and/or extensive side effects etc. If there is no suitable technology, we investigate and create a new pharmaceutical technologies, i.e., a novel invention. We hear voices and needs of patients and medical professionals from other departments members in such as sales reps, and we confirm what kind of formulation is required.
We prepare and evaluate prototype DDS formulations in vitro and in vivo to confirm their performance. These evaluations are conducted by ourselves, and sometimes by research division members with specific techniques. After execution of cycles of the studies to improve formulations performance, we file formulation patents application if the concept of DDS technology is confirmed.
To realize the prototype DDS formulation into “drug products” having higher quality, stable manufacturing, we relay the baton to researchers of Formulation Design Laboratory and CMC Research. They investigate optimization of formulation compositions, such as types and amounts of excipients, and manufacture investigational drugs for clinical trials. To establish stable and efficient manufacturing process for the high-quality “drug products” with low cost, researchers at Process Technology Laboratory optimize the process, and transfer the technology to global manufacturing sites. To protects the quality of “drug products” during long-term storage and transportation, researchers at Packaging and Devices Laboratory design the optimal packaging which is easy to use for patients and medical professionals. In recent years, we also develop administration devices for easy dosing and medical apps (Software as Medical Device).
In addition to experiments and collaboration with various experts, search and understanding of the world's cutting-edge DDS technologies are also important aspects for us. Reading the latest and cutting-edge papers and patents enables us to select the most suitable DDS technology for each modality and issue and promote development of drug products. -
When do you find work interesting or difficult?
Research often does not progress as expected, but I feel pleasure of being able to quickly deliver high value to patients, and scientific interesting when we obtain experimental data which contribute to projects promotion even a little through trial and error with team members.
In some projects, we discuss with overseas members in English. Previously, when I proposed a future plan based on our data, miscommunication prevented smooth discussion and obtaining consensus. Therefore, I obtained additional data, improved phrasing and expression, and finally, I could get consensus on our idea and felt a sense of accomplishment. -
In your work, what challenges would you like to take on in the future?
I would like to try formulation development for various “drug seeds” and deliver the value to patients.
Issues to be solved and the suitable formulations depend on the modality of “drug seeds''. Content of investigation and collaboration partners also differ between drug discovery projects in early stage and development of additional dosage forms for launched products as LCM (life cycle management). In order to make drug products of the various “drug seeds” launched, I intend to continue to challenge technology search and formulation preparation, accumulate knowledge and experience, and would like to contribute to “value for patients'' as a formulation researcher.
Moreover, expertise in many fields is essential to promote projects. For commercialization of products, it’s extremely important to communicate with a variety of key stakeholders having expertise such as pharmacology, synthesis, pharmacokinetics, safety, regulation, stability, equipment etc.. Mutual understanding is also a key for success in collaboration with academia and startups.
I would like to learn more knowledge from my seniors, expand my views as a pharmaceutical scientist, and continue to take on challenges together with colleague teams. -
What is the appeal of working at Astellas?
We can feel our own growth while receiving cooperation and support from many colleague.
As one of attractive points in working with Astellas, we, young researchers, can be assigned to a responsible researcher of a project, receive generous support from our senior colleagues, and grow ourselves through the projects. In organizational culture of Astellas, employees share own thoughts and ideas and respect each other, regardless of age or department.
In my second year working in Astellas, I was assigned as a responsible researcher of a project for the first time, and I didn't know what/how to proceed with the project and how to collaborate with colleague etc. At that time, I received many advice and suggestions from my seniors and team members, and tried candidate ways by myself, and gradually I was able to proceed the project more smoothly. Even now, I sometimes consult with my seniors when I don’t have confidence in selection of options. Senior colleague always take time to listen to my opinions and give me thoughtful advice even in their busy time. I believe, another attractive point in working with Astellas is that we can respect many seniors not only as a researcher, but also as a person.
Additionally, Astellas has many programs for employee’s growth. For example, young colleague, called mentors, teach the basic social manners and company rules to new commers (one and a half years after joining Astellas). To encourage growth as scientists, there are several supporting systems such as obtaining Ph.D. degrees, participation in academic conferences, and visiting scholars at foreign academia.
I will continue to grow myself day by day so that I can become a reliable senior for my juniors.
An example of my daily schedule
| 8:30 to 9:00 | Come to the office. Check e-mails and confirm my schedule for the day. |
| 9:00 to 10:00 | Discuss experimental data with team members to determine our future plan. Sometimes the discussions are in English. |
| 10:00 to 12:00 | Do experiments. Check e-mails during breaks. |
| 12:00 to 13:00 | Lunch with department members at the cafeteria in the research center. Relax and enjoy playing table tennis with everyone. |
| 13:00 to 14:30 | Do experiments. Check e-mails during breaks. |
| 14:30 to 16:30 | Read scientific papers. |
| 16:30 to 17:30 | Summarize experimental data and prepare presentation materials for meetings. |
| 17:30 to 18:00 | Check e-mails and schedule for the next day, and then I go back home. (Time to go home is different depending on the day, and sometimes I go home earlier. Since business hours is 8:30-15:45 on every Friday, I enjoy playing tennis with my colleagues at the company tennis court around 16:00.) |
* Affiliation at the time of posting.
Analytical Research Laboratories
Overview
The Analytical Research Labs. supports Astellas' "MONOZUKURI" technology development in terms of quality
through high-level physico-chmical/biological and analytical research based on physical chemistry,
structural chemistry, biology, and analytical chemistry from the early stages of drug development to
post-marketing stages, in order to deliver highly functional and appropriate quality drugs to patients.
Specifically, we develop test methods, set quality standards, and conduct stability evaluations based on
research into the physicochemical, structural chemical, and biological properties of active
pharmaceutical ingredients (APIs) and their formulation characteristics. In addition, we transfer the
technology of test methods developed by the Analytical Research Laboratories to the testing departments
at domestic and overseas testing sites and manufacturing sites, and provide analytical technical support
by offering more optimal test methods throughout the product life cycle.
The network of the Astellas Analytics is expanding, centered on the Analytical Research Laboratories,
Astellas US Technologies Inc. in the US, and Astellas Pharma Europe B.V. in Europe, with researchers
working on a global basis.
Furthermore, they prepare analytical and quality-related technical documents for clinical trial
applications and new drug applications in various countries around the world, and work with regulatory
affairs departments in Japan and overseas to handle the approval review process, as well as responding
to applications for changes after approval. As the number of countries subject to regulatory approval
expands and pharmaceutical regulations strengthen globally, we aim for early approval by conducting
highly reliable, scientifically based physical property and analytical research. In particular, we are
introducing new analytical research concepts such as Analytical Quality by Design (AQbD), a new
analytical technology based on Chemometrics, and utilizing data science, as well as developing cell
based assays and functional assays for biopharmaceuticals. For biopharmaceuticals, we are actively
engaged in the development of cell-based assays, functional assays, and characterization methods using
high-resolution LC-MS.
Main research themes
Development of new analytical methods, application of new technologies to pharmaceutical analysis, and
solution of problems in APIs/formulations through scientific approaches
Analytical research on APIs; physical property research, test method development and specification
setting, stability evaluation, etc. to support process development of APIs
Analytical research of pharmaceutical formulations; physical property research, test method development
and specification setting, stability evaluation, etc. to support formulation research, process
development and packaging design development
Technology transfer of test methods and maintenance of analytical technology to the testing departments
of group companies, contract testing companies outside the group, and manufacturing site testing
departments inside and outside the group.
Preparation of submission materials for Japan, the U.S., Europe, and other emerging countries, and
handling of approval reviews and change applications
Development of cell based assays and functional assays for biopharmaceuticals, characterization and
method development using high resolution LC-MS, and genome analysis using NGS, etc.
Introduction and development of new analytical techniques based on chemometrics, new analytical research
concepts such as AQbD, and data science-based methods for investigating the causes of quality issues in
CMC research.
Robotics for CMC research and study of automation of quality testing at test sites.
Message from Vice President

Kei Motonaga
The function and quality of a pharmaceutical product are not simply expressed by the analytical
results of the final product, but are created through a research process that determines relevant
properties throughout the research and development of the drug substance and drug product, and leads
to more sophisticated process establishment, formulation design, and packaging design. The name
"Analytical Research Labs." is derived from the concept of "scientifically exploring the critical
properties of products".
The scope of our work ranges from the initial development stage to the end of the product's lifecycle.
The countries where applications are filed and the production sites are increasingly globalized.
Research and development (R&D) targets are not limited to active ingredients such as viruses, cells,
antibodies, and chemically synthesized pharmaceuticals, or to their formulations themselves, but also
include pharmaceutical excipients such as functional polymers, which require various characterization
technologies as formulation and device functionnalites become more and more sophisticated. The
Analytical Research Labs. is open to all researchers, regardless of their field of expertise, who wish
to find their own dream in bringing valuable medicines to the patients in the world.
Employee Q&A

I develop analytical methods to evaluate the quality of drug products of small molecule and conduct research to understand their physico-chemical properties.
Joined in 2021, graduate of master's degree at the Graduate School of Medical and Pharmaceutical Sciences (Pharmaceutical Sciences)
Hitomi Okada
-
What is your current job?
In order to deliver highly functional, high-quality drugs to patients as quickly and stably as possible, I am engaged in the development of test methods and research on the physico-chemical properties of drug products.
Proper design and manufacturing of drugs and assurance of their quality are essential to deliver safe and reliable drugs to patients. In the Analytical Research Labs., we mainly develop test methods to evaluate the quality of drugs (drug substances and drug products) and conduct research to understand their physico-chemical properties.
I am currently in charge of the development of test methods and research on the physico-chemical properties of small molecule drug products (formulated products for administration to patients) under development.
In the test method development, we control the quality of drug products by setting test procedures and acceptance criteria from the development stage when clinical trials are initiated. As the development progresses, the level of control is increased to achieve higher quality in terms of efficacy, safety, and stable supply. A drug product is not a single component of the drug substance (the active ingredient of a drug), but a mixture including excipients. Drug product-specific impurities resulting from reactions with excipients and packaging can be present in the drug product. Furthermore, a single drug substance can be used to design drug products with various contents, dosage forms, and formulations. Therefore, when developing a test method for a drug product, sample preparation methods and analytical conditions must be optimized according to the properties of the drug product, and a wide range of test items must be set.
In physico-chemical properties research, we evaluate the functionality of formulations, such as improvement in stability and dissolution by excipients, and investigate the causes of quality issues. The information obtained from the physico-chemical properties research is shared with members responsible for formulation design and used to develop and improve formulations and manufacturing processes, thereby contributing to Astellas’s entire process of creating drug product from the quality aspect.
In addition, I prepare documents for clinical trial application in various countries, transfer test method technology and outsource stability tests to analytical sites inside and outside the company, and conduct basic research for understanding drug product properties and exploring new technologies. -
When do you find your work interesting or difficult?
I find it interesting to come into contact with a wide variety of knowledge and technologies, and to utilize the skills and know-how I have acquired to move forward with my work.
For the development of a single drug product, members with various backgrounds, including synthesis and analysis of drug substances, formulation and process design, work together. Furthermore, a wide range of technologies are used. In order to correctly understand and evaluate the quality of a drug product, not only knowledge of the physico-chemical properties analysis of the drug product and instrumental analysis techniques, but also knowledge in other specialized areas is necessary. Since joining the company, I have been given opportunities to develop various test methods, conduct research on physico-chemical properties, and attend meetings and training sessions with members from other specialized fields. Through these opportunities I feel that my knowledge and skills are expanding every day. I feel a sense of accomplishment when I am able to develop and present optimal analytical methods in accordance with the characteristics of the analyte based on my accumulated knowledge and experience.
In some cases, problems with test methods and analysis, such as a lack of reproducibility or observation of outliers, could occur. It is sometimes difficult to solve the problems because the root cause could not be immediately identified. Even in such situations, I find it interesting to gain new insights through discussions with my seniors, information gathering from other departments, repeated experiments, and trial-and-error. When we reach a solution, I feel a great sense of accomplishment, and at the same time, I feel a sense of growth, which is very rewarding.
In order to work efficiently and effectively, knowledge of pharmaceutical regulations, knowledge of statistics and data science for analysis, and an understanding of the needs of analytical sites for technology transfer and outsourcing are also required. I feel that I have a lot to learn in my research life, and I am motivated every day by the fact that every single task I accomplish with the know-how I have gained is connected to "delivering drugs to patients". -
What is something you would like to challenge yourself in the future at work?
I would like to acquire a wide range of expertise centered on physico-chemical properties analysis, and to be able to lead the project in cooperation with people who are active in other fields.
The work of the Analytical Research Labs. involves various processes from the development stage to post-launch. Currently, I am involved in the project of small molecule solid drug product in the early stages of development. In future, I would like to enhance my expertise in physico-chemical property analysis while gaining experience by challenging myself at various stages and analytical target. Like my seniors, I would like to be able to proactively lead the projects and contribute to the other departments from the viewpoint of physico-chemical property analysis.
In addition to enhancing my expertise, I would like to take on challenges of introducing new technologies and proposing new solutions by expanding my knowledge and skills in other related fields and refining my imagination, creativity, and ability to think critically. I would also like to deepen my understanding of the work in different fields and contribute to promoting effective collaboration that takes advantage of each other's strengths.
Furthermore, I am currently actively involved in joint research with other research institutions and participating in academic conferences, which gives me many opportunities to look outside the company. My dream is to continue to participate not only in internal but also external activities and eventually become a researcher who can contribute to the pharmaceutical industry as a whole through physico-chemical property analysis. -
What attracts you to working at Astellas?
I am attracted to the environment where I can grow and take on challenges.
Astellas has a culture that respects diversity and individual personality, and there are people with various backgrounds, specialties, and careers inside and outside the research laboratories. Moreover, as a global pharmaceutical company, Astellas has many relationships with people from overseas. In e-mails and meetings, we have lively discussions from various perspectives. I feel that I am inspired and broadening my perspective in the global stage. Another attractive aspect is the opportunity for employees to share and enhance each other's knowledge, experience, and strengths through work-related study sessions and debriefing sessions. Furthermore, the company values and respects the opinions of young employees. We are blessed with an environment where young employees are entrusted with important work and encouraged to actively take on new challenges.
In the Analytical Reserach Labs, we have working team (WT) activities to conduct basic research for a deeper understanding of physico-chemical properties and to develop technologies to solve common issues among new drug development projects. I have participated in several WT activities. One of the attractive points of WT activities is that there are opportunities for growth as a researcher, where we can try the development and acquisition of new technologies based on our own ideas without being bound by precedents.
A Day in the work of Hitomi Okada
| 6:30 | Wake up. It takes about 15 minutes to walk from home to the office. |
| 8:00-8:30 | Arrive at work. Check e-mails and the daily schedule. |
| 8:30-9:00 | Analyze the results of the previous day's experiments. |
| 10:00-12:00 | Conduct experiments. In my spare time, check e-mails and prepare documents (most of the documents are prepared in English for the global market). |
| 12:30-13:30 | Have lunch at the company cafeteria. |
| 13:30-14:00 | Discuss experiment details and documentation policies with team members. |
| 14:00-15:00 | Attend meetings with other departments (some meetings are with overseas sites). |
| 15:00-17:00 | Continue experiments and record experiment details in the electronic laboratory notebook. |
| 17:00-18:30 | Prepare reports and meeting materials. |
| 18:30 | Confirm the schedule for the next day. Leave the office (leaving time may vary depending on the day). |
Employee Q&A

I develop analytical methods to evaluate the quality of biopharmaceuticals and conduct research to understand their physical properties.
Graduate School of Pharmaceutical Sciences, Division of Bioinformatics and Chemical Genomics, Master's Degree, Joined the company in 2020
Shintaro Yamane
-
What is your current work?
I am engaged in the development of analytical methods for evaluating the quality of drugs and research to understand their physical properties.
I am mainly engaged in the development of analytical methods and research on physical properties related to the evaluation of biological activity of antibody drugs under development. I am developing analytical methods to evaluate the quality of developed antibodies based on the mechanism of action, such as the binding between antibodies and antigens, and the signal transduction reaction associated with the binding. We use various methods such as ELISA, flow cytometry, and reporter gene assays. We are working on understanding the mechanism of action and examining the test conditions in order to develop analytical methods that can correctly and stably evaluate the activity. The analytical methods we develop are used as part of quality testing to ensure that the manufactured drug product meets the desired quality.
In addition, I conduct research to understand the properties and characteristics of developed antibodies as part of our physical properties research. Analysis of the structure of developed antibodies, stress-induced structural changes, and the correlation between such structural changes and biological activity are analyzed to gain a better understanding of developed antibodies, to select important parameters for quality control, and to provide justifications to pharmaceutical regulatory authorities. The research is carried out in conjunction with structural evaluation information obtained by HPLC and mass spectrometry (MS) at the same laboratory, so a wide range of knowledge is required. I think that elucidating physical properties based on various data is a very interesting part of the research. Of course, collaboration with other laboratories is also indispensable in understanding physical properties. The data on the physical properties of developed products obtained from the manufacturing process research conducted by the Chemical & Biological Technology Research Laboratory and the formulation research conducted by the Pharmaceutical Research & Technology Laboratories is valuable information that cannot be obtained by the Analytical Research Labs. alone. I analyze samples produced in the research process at each laboratory to obtain data on physical properties and provide the analysis results for each laboratory to promote research at each laboratory from the viewpoint of physical properties. -
When do you find your work interesting or challenging?
The process of developing robust analytical methods is very difficult and interesting.
The various analytical methods we develop at our laboratory are used for long-lasting quality testing at manufacturing plants in Japan and overseas. Therefore, they must be robust analytical methods that can evaluate the quality of pharmaceuticals at a certain level regardless of the testing site or the analyst. When developing an analytical method, we investigate factors that may affect robustness, such as the source and lot-to-lot difference of reagents and the manufacturer and model number of analytical equipment, in addition to the operating procedures, analysts, and testing sites, to optimize the testing conditions. In particular, it is very difficult to obtain stable data for cell-based test methods because the analytical results may vary due to changes in cell conditions caused by slight changes in culture conditions or differences in passage number. For this reason, I feel a truly great sense of accomplishment when I understand the characteristics of an analytical method and complete a robust test method after much trial and error.
Another attractive point is that the work of the Analytical Research Labs. is very broad. In addition to the microplate reader and flow cytometer that I am currently using, there are HPLC, GC (and other chromatographic instruments), MS, NMR, DSC, X-ray equipment, and etc. When I was a student, I mainly conducted bioactivity evaluations using cells, but since joining the company, I have also been conducting analytical method development using HPLC, MS and other instruments that I have never used before, so I am learning new fields through work.
In addition to experimental work, we also handle regulatory affairs application and transfer analytical method technology to domestic and overseas testing sites, so I find it very interesting and challenging to work at the Analytical Research Labs., where I am constantly learning new knowledge and technologies. By learning new fields, I am able to gain a deeper understanding of drug development, which always keeps my days fresh and enjoyable. Of course, when learning a new field, I can take on the challenge without worry as my senior experts provide detailed guidance. -
What would you like to challenge yourself in the future at work?
As an analytical specialist, I would like to be able to lead development projects.
As mentioned above, the work of the Analytical Research Labs. requires a wide range of skills, including analytical method development, physical properties research and pharmaceutical applications. Therefore, my daily work is a series of challenges. In the future, I would like to acquire a wide range of know-how in various fields by accumulating these daily experiences, and to be able to lead projects as an expert in analysis. In addition, as a project leader, I would like to contribute to the stable supply of safe and trusted drugs by collaborating with various departments within our company and with manufacturing and testing companies in Japan and overseas. Although I am still a novice in every field, I feel that I am growing step by step as I gradually gain experience in developing various types of test methods, acquiring data on physical properties, and preparing regulatory applications. I would like to continue to take on new challenges and continue to grow.
-
What is the attraction of working at Astellas?
It is the excellent training system and growth support for employees.
We have a mentor system in which you work together with senior employees for the first year and a half after joining our company. They are always available for consultation, so you can work with peace of mind. Of course, other than the mentors, all senior employees are also kind enough to teach you. During discussions, they are always willing to treat each other as equals as researchers.
Astellas is a "treasure house" of experts in various fields, so to speak, and I feel that it is an environment in which it is easy to take on challenges in new fields.
The Analytical Research Labs. is eager to acquire new technologies and research methods, and even young employees are given opportunities to actively take on new challenges. Since my third year with the company, I have joined a new technology development team on a voluntary basis, in addition to my work on development projects, and am working on the establishment of a test method to evaluate immunogenicity which is related to the safety of drugs.
There are also various other opportunities for businessperson to obtain doctoral degrees, study abroad or be posted overseas. The will to grow with the company as well as yourself is respected, and your supervisor actively encourages you to do so.
I feel that Analytical Research Labs. has a particularly good support system. The phrase that left the greatest impression on me during my job search was, "Analytical Research Labs. is a department that values human resources."In fact, few people have the experience in the development of high-precision analytical methods, extensive evaluation of physical properties and pharmaceutical application work that is performed at Analytical Research Labs. during their university days. For this reason, the department as a whole focuses on "nurturing employees," which I feel leads to the enhancement of training programs and growth support.
I am also attracted to the fact that I can grow on a global stage. Astellas has R&D and production sites in Japan and overseas. The projects I am currently in charge of are all global development products, and I sometimes have meetings with overseas representatives in English. In order to obtain regulatory approval in various countries, we file regulatory applications in accordance with country-specific regulations. I feel that the appeal of working at Astellas is that I can broaden my horizons through this kind of work in the global arena.
A Day in the work of Shintaro Yamane
| 7:30 | Wake up. Commute to work takes about 15 minutes by car. |
| 8:30 - 9:00 | Arrive at work. Check email and daily schedule. |
| 9:00-10:00 | Weekly bioactivity evaluation team meeting (members who mainly conduct bioactivity evaluation gather to report on the status of their experiments and discuss what other members are studying). |
| 10:00-12:00 | Experiment (cell passaging, start of cell tests). |
| 12:00-13:00 | Lunch at the cafeteria in the laboratory. On a fine day, I take a walk around the laboratory with my colleagues to refresh myself. |
| 13:00-15:00 | Experiment (measurement with FCM), analysis of experimental results. |
| 15:00-16:00 | Discussion with senior employees about the results of experiments and future studies. |
| 16:00-17:00 | Monthly joint project meeting with other laboratories (sharing the analysis results of research samples from other laboratories and discussing the results and future study policies). |
| 17:00-18:00 | Preparation of progress report materials and reports on experimental details. Occasionally, literature research is also conducted. |
| 18:00-18:30 | Confirm the next day's schedule and leave the office (leaving time may vary depending on the day). |
Engineering Group
Overview
The Engineering Group works on "engineering" and "technology development" activities. In both of these tasks, we work to optimize the entire procedure based on environmental, safety, quality, delivery time, and cost perspectives. We accurately incorporate the requirements of users who are in charge of in-house research divisions and manufacturing plants in the specifications of equipment, and work with external engineering companies and equipment manufacturers to build facilities and equipment. In addition to maintaining close contact with the internal Quality Assurance Division and Procurement, we also maintain close contact with regulatory authorities, and work closely with a large number of related divisions during these construction activities.
Engineering tasks
We construct new buildings, build manufacturing lines, refurbish existing
buildings, and introduce new technologies. In recent years, operational plans that take into account
the entire lifecycle, from the design of facilities and equipment to construction and installation,
testing operations, actual operations, and retirement, have been required. By taking these into
consideration from the design stage, it is feasible to construct more advanced specifications for
facilities and equipment. This is an example of the capabilities of engineers, because how deeply the
entire lifecycle has been considered at the design stage can have a significant impact on subsequent
operations.
Plant construction: We design and construct manufacturing plants. In the past, we built numerous
plants for chemical synthesis of drug substances and oral solid dosages for batch production.
Recently, in addition to these, we have also built manufacturing plants for antibodies and new
modalities for pharmaceutical products. We design and adopt facilities and equipment while meeting new
regulatory requirements.
Construction of manufacturing lines: In order to build new product manufacturing lines and increase
manufacturing capacity, we introduce manufacturing equipment in future spaces at existing facilities
and renovate existing lines. In addition, in the event of aging facilities, we are not simply
replacing equipment, but are introducing equipment with new highly functional technologies. To
introduce these new technologies, we also conduct technology evaluation experiments in collaboration
with various research laboratories.
In plant construction and manufacturing line construction, we pay due attention to the environment. We
work to build facilities and equipment that are energy efficient by introducing highly efficient
equipment and systems so that manufacturing and research activities can be conducted with less energy.
Technical development operations
Quality improvement technologies
Manufacturing equipment technology plays an extremely important role in ensuring
and improving the quality of pharmaceutical products. In order to improve the quality of
pharmaceutical products, the Engineering Group develops and introduces various manufacturing equipment
technologies.
For example, the establishment of a production line for a new pharmaceutical product requires
equipment that will ensure that the quality characteristics for the new product are met; however, such
equipment may not exist in the market and, in such instances, we need to combine existing technologies
or develop new equipment ourselves. Although the quality of pharmaceutical products has been ensured
by taking samples from each process and performing quality tests after the completion of the process,
developing technologies to directly monitor and manage the quality of products during the process is
one example of our developments.
Automated technology
Automated technologies are very important for pharmaceutical products not only to
reduce the cost of manufacturing but also to avoid the risk of contamination by humans. The
Engineering Group collaborates with external device manufacturers in the development and introduction
of technologies for automation of pharmaceutical production lines based on the latest control and
robot/IT technologies.
For example, we can automate testing tasks by introducing testing machines, and we can automate
material handling tasks by introducing AI/robots
Digital technology
In the research, manufacturing, maintenance, and other various operations of the Pharmaceutical Technology Division, we seek ideas within the company of things we "wish were possible," and are working to investigate digital technologies to actualize such ideas and integrate them into equipment and instruments. Most recently, we applied mixed reality (MR) technology to the current manufacturing and analysis operations, with the goal of achieving more accurate and efficient operations.
Main themes of activities
Domestic and overseas construction projects for research laboratories and manufacturing facilities for
commercial produces and clinical trial materials
Establishment of new manufacturing lines: work to design and introduce manufacturing lines for new
products or new dosage forms
Work to introduce research equipment: work related to the design and introduction when newly installing
or renewing individual equipment used in research laboratories
Development of new technology listed below.
Development and introduction of technology to monitor and control the quality of pharmaceutical products
in real time
Development of testing machines: work to develop and introduce testing machines to ensure the various
qualities of pharmaceutical products
Development of automation technology: development and introduction of technology that automates human
tasks using machines
A Message from the Group Leader

Hideyuki Tomoda
When you hear the word "engineering" in a pharmaceutical company, few people have
an immediate image of it.The main task of the Engineering Group is to create "research facilities and
plants where pharmaceutical products are manufactured." However, we do not draw plans or operate
machinery.Our work is to clearly requirements and functions that research facilities,
plants/factories, various equipment and systems,need to have installed, and after communicating and
discussing these with expert equipment manufacturers and engineering companies, we put the
requirements into practice. In general, equipment manufacturers complete their tasks after they have
delivered the equipment and confirmed normal operations, and engineering companies complete their
tasks after the plant is completed and handover is complete. From there,This is the most important and
rewarding part of our work.This is because it is only often possible to confirm whether products are
being manufactured at the quality required by users, whether there are any issues with workability and
the flow of people and products, etc., after these have been put into operation. As an engineer, it is
a great pleasure to see the equipment working as originally intended and to see our users happy. To
this end, it is important that we ensure we have an understanding of the intentions of researchers and
plant staff, who are our users, and integrate them into equipment/facility requirements. This work can
be compared to an interpreter in the sense of translating scientific knowledge into engineering
knowledge. We strive to be good translators every day.
The expertise required in engineering is
wide-ranging.The basic focus is on construction, air conditioning, electricity,hygiene, and utilities,
etc. In the development of new technologies, various specialties are required from perspectives other
than facilities, such as considering the products, substances, and handling.As a result, we have
engibeers with diverse background and expertise.Work is assigned in accordance with skills: first
introducing small equipment, then building a mid-size manufacturing line, and then a large plant.Due
to the frequent regulation changes and requirements, we share what we do not know and work together to
grow, regardless of age or career, and we work to grow together.In addition to work at our domestic
plants and laboratories,we are also in charge of the work at overseas plants. There are also
opportunities to play an active role globally. Join us in an engineering group that contributes to the
health of people around the world through our engineering knowledge.
Employee Q&A

Master of Process Engineering, Molecular Chemistry and Engineering Course,
Graduate School of Chemical Sciences and Engineering
Joined the company in 2017
Natsumi Hasegawa
-
What is your current job description?
The main missions of the Pharmaceutical Technology Division include "transforming the drug seeds discovered by the drug discovery research division into a form that is available for patients to take," "ensuring a stable supply of medications is available for patients to take," and "building the technical infrastructure to support the various forms of treatment that will be developed in the future."
Within this scope, the Engineering Group is contributing to the achievement of the Pharmaceutical Technology Division’s mission by installing equipment for manufacturing investigational products to confirm the efficacy and safety of the pharmaceutical products and commercial pharmaceutical products that can be prescribed at pharmacies and hospitals, working on construction projects at plants, research laboratories, and other facilities to manufacture pharmaceutical products, and developing new technologies to improve the quality and manufacturing efficiency of pharmaceutical products. In particular, these days the speed of technological innovation, such as the use of new therapies and new modalities, is accelerating, so it is necessary to establish appropriate facilities and technologies. We work in collaboration with individuals with a wide range of expertise.
The main work I am currently involved in is building a line for manufacturing injectable formulations for clinical trials, which is designing, verifying the operation, and adopting manufacturing equipment. When designing equipment specifications, we work in collaboration with individuals within and outside the company. Since the facility design phase has been advancing recently, we received plans from equipment manufacturers reflecting the contents of discussions to date, and are proceeding with further discussions. -
When do you find work interesting or difficult?
I find it both interesting and challenging when it comes to needing to collaborate with individuals from a broad range of departments within and outside the company, and when it comes to needing to have broader knowledge.
As for those involved in our work, we work with many parties outside the company, including construction companies and various equipment manufacturers and, within the company, we work with various divisions including the research, procurement, and manufacturing divisions. During this process, we determine the specifications of the equipment to be installed, but there are times when opinions conflict and discussions do not proceed as well as we would like, so I feel that there are some difficulties as a coordinator. On the other hand, while hearing the opinions of various individuals, I also have the opportunity to come into contact with ideas and knowledge that I could not come up with, so I often find it interesting.
In addition, in terms of the knowledge required for our work, it is necessary to make use of a lot of knowledge and experience, such as various regulations and understanding the specifications of equipment to be introduced, even in a single project. Furthermore, there are various matters that need to be considered because, in addition to the differences of regulations in each country, there are manufacturing facilities, utility facilities, and system equipment that need to be introduced, and it is also necessary to clarify facility specifications for buildings, air conditioning, electricity, etc. Moreover, I feel that there is no end to what we need to learn because we manufacture different products, such as drug substances and drug products (oral solid dosages, injectable formulations, etc.), depending on the manufacturing site. It takes energy to fight this, but I also find it interesting to be exposed to new things. -
In your work, what challenges would you like to take on in the future?
What I would like to do in the future is learn about forms of pharmaceutical products and treatments that are different from those used in the past, and participate in construction projects in different phases.
For the former, the manufacturing process of drug products can be roughly divided into two processes: the drug substance process for mass production of drug seeds, and the formulation process for converting the drug substance into the form of the drug product. So far, I have been involved in the manufacturing process for commercial tablets of the drug product, and the manufacturing process for injectable formulations for clinical trials. On the other hand, as an area where I have no experience, I would like to take on the challenge of constructing manufacturing facilities for new modalities, including cell preparations. In the Engineering Group, there are opportunities to take on a wide range of tasks, and it is an environment that makes it easy to take on challenges.
Also, in a construction project, after the concept of the facility to be constructed is determined, the basic design is devised, and then the detailed facility and equipment specifications are established. In the past, I have often been assigned to projects after the completion of the concept design of the project, so I also want to be involved in the project from an earlier stage. -
What is the appeal of working at Astellas?
The attractive points of working at Astellas are that through building a pharmaceutical manufacturing facility, I am able to contribute to the health of many patients and be involved in the large scale of work.
Astellas' management philosophy is to contribute to the health of people around the world. I rarely have the opportunity to interact directly with patients, but I do feel that my work is significantly contributing to the health of people around the world. Also, the project that I am currently involved in is a project for which a budget of approximately 18 billion yen is being invested in conjunction with the commercial drug manufacturing line that is ongoing at the same time, and thus, I feel that I have been able to be involved in large-scale work from an early age in terms of costs.
In the department where I currently work, including for the current project, there is a tendency for young employees to actively take on important tasks, and I find it appealing to be able to experience growth. In actuality, before I was involved in building the current injectable formulation line, I had the opportunity to travel on a long-term business trip to the site in Toyama Prefecture for half a year and acquire the skills to manufacture sterile products.
A day in the life of Natsumi Hasegawa
| 6:00 | Get up. I go from my home to the office by train, including transfers. |
| 8:15 to 9:00 | Get to work. Check e-mails, confirm the schedule for the day, which was organized on the previous day, and tack on add-ons. |
| 9:00 to 10:00 | Participate in regular on-site meetings as part of a construction project. Collaborate internally and externally on design progress, internal requests associated with construction, and progress in meeting timelines |
| 10:00 to 12:00 | Participate in manufacturing subcommittee meetings as part of a construction project. Be conscious of making appropriate internal decisions on equipment specifications in meetings that include both internal and external parties, and proceed with the meeting while providing necessary additional information. |
| 12:00 to 12:30 | Organize issues raised in the manufacturing subcommittee meeting and compile the materials necessary for internal agreement. |
| 12:30 to 13:30 | Lunch at the company cafeteria. There is a large menu, and I enjoy choosing from the menu every day. |
| 13:30 to 16:00 | Continuing from the morning, participate in manufacturing subcommittee meetings. Move forward with determining specifications for different equipment than that from in the morning. |
| 16:00 to 17:15 | Organize issues raised at the afternoon manufacturing subcommittee meeting and compile the materials necessary for internal agreement. |
| 17:15 to 18:30 | As part of the introduction of a single unit of equipment for investigational product manufacturing, put together materials related to equipment specifications. Since this is an equipment replacement , steps are taken to make it easy for workers to use based on the current equipment, and items to be checked with the manufacturer are listed while gaining an understanding of the points to achieve the required level of product quality. |
| 18:30 to 19:00 | Check e-mails, organize my schedule for the next day. |
| 19:00 | Leave work (the time I leave work varies depending on the workload. On Fridays, there is a Family Friday system where people can go home around 15:30 to 17:00.) |
* Affiliation at the time of posting.
Employee Q&A

Master of Bioengineering, Mechanical Science and Bioengineering Course,
Graduate School of Engineering Sience
Joined the company in 2019
Masaki Taneo
-
What is your current job description?
In my current role, my primary focus is on enhancing the quality and efficiency of drug manufacturing by utilizing the latest technologies such as AI, XR, RFID, biometrics, and robotics.
Since joining the company, I've been primarily involved in technical development, collaborating with various departments in the pharmaceutical technology sector to explore the feasibility of implementing these technologies in drug manufacturing processes. This encompasses a wide range of activities beyond pharmaceutical production itself, including maintenance, technology transfer, inventory management, access control, and laboratory research.
The pharmaceutical industry is often said to lag behind other industries in the adoption of new technologies due to the high demand for safety in providing products that involve human lives, and the strict rules that must be adhered to in drug development. Within such constraints, there is a strong demand for agility and flexibility to quickly verify the effectiveness of new technologies and proceed with their implementation to the extent possible.
Currently, one of my key projects involves validation of AI predictive maintenance system.This system utilizes AI to detect abnormalities in equipment in advance, using data collected from the equipment to conduct machine learning and verify whether past abnormalities could have been predicted. We're working on this with a focus on eliminating sudden equipment failures, contributing to the VALUE for patients by ensuring a stable supply of medications. In addition, we regularly participate in exhibitions to collect new technical information. -
When do you find work interesting or difficult?
I find it both interesting and challenging because I have to collaborate with a diverse array of people across various departments, both internally and externally, and because of the significant flexibility in my role.
Especially when I meet with manufacturers who own new technologies, I often find it challenging to realize that assumptions one party makes might not align with the other's.
Moreover, technology development involves a lot of flexibility, requiring individuals to set their own criteria for verification and final goals. I find it interesting to have some degree of discretion in approaching tasks, but at the same time, I feel the difficulty in setting goals during the early stages of projects is crucial. -
In your work, what challenges would you like to take on in the future?
What I would like to try in the future is participating in construction projects.
Since joining the company, I have mainly been engaged in technology development work, partly because it was my wish.As for engineering projects, I have only been in charge of basic design work for equipment introduction and renovation projects that do not involve development elements, and I have no experience in actually building buildings or conducting renovation work. Therefore, I would like to gain experience in engineering work at the construction stage in the future.This is because as we proceed with technology development work, engineering knowledge and experience are often required, and I feel that experience in both, rather than one or the other, is necessary. In particular, the launch of a new building can be properly called "manufacturing" because it visibly creates something from nothing. If I have the opportunity, I would like to participate in the project to launch the new building.
-
What is the appeal of working at Astellas?
In my current department, younger employees are often given significant responsibilities early on. In my second year, I took the lead on renovation planning and basic design for a section of our investigational drug building. By my third year, I was managing multiple projects related to new technology development, collaborating across departments. Although I had senior colleagues and supervisors for guidance, I primarily managed these projects independently, handling everything from scheduling to implementation. This allowed me to gain diverse experiences and feel a strong sense of personal growth. Another aspect of Astellas that I find appealing is its culture of embracing challenges company-wide
A day in the life of Masaki Taneo
| 7:00 | Wake up.Commute by car takes 30 minutes. |
| 8:30 to 9:00 | Arrive at the office. Check emails and review the schedule for the day. |
| 9:00 to 10:00 | Meeting with internal counterparts to discuss ongoing technical development projects. |
| 10:00 to 12:00 | Reviewing verification items and preparing documents for technical development. |
| 12:00 to 13:00 | Lunch break. |
| 13:00 to 15:00 | Meetings with external manufacturers and agents. |
| 15:00 to 16:00 | Internal meetings. |
| 16:00 to 18:30 | Document preparation. |
| 18:30 to 19:00 | Check emails and organize schedule for the next day. |
| 19:00 | Leave the office (Note: Departure time may vary depending on workload. On Fridays, there is a Family Friday program, so departure may be around 4:00 PM). |
* Affiliation at the time of posting.
CMC Research
Overview
In the drug discovery stage, we contribute by preparing samples necessary for drug discovery and evaluating the physicochemical properties of development candidates, we also use the knowledge gained there to make process development for the clinical stage, and we tackle to accelerate new drug discovery. We have also developed and established technologies and intellectual property that are essential for commercializing candidates found in drug discovery. The work of early CMC development is wide-ranging, as described below. For this reason, CMC Research members are mainly assigned from professional researchers trained in each CMC Development. More specifically, we have members with a wide range of expertise, including dryg substance, drug product, analysis, manufacturing, and regulatory affairs. By efficiently integrating the high MONOzukuri technology cultivated in each specialized field within the same organization, we can proceed with development with high consistency and speed, even for commercialization of new modalities with little experience.
Main research themes
Drug substance and drug product manufacturing process development research
Formulation design for initial clinical studies
Development research of testing methods to evaluate the physical properties of development candidate products in the drug discovery research stage and physical property evaluation
Manufacturing and supply of drug substances and drug products for clinical studies using in-house equipment and CMOs
Development research of testing methods to evaluate the quality of drug substances and drug products for clinical studies and quality evaluation
Transfer of manufacturing processes and quality testing methods for drug substances and drug products to CMOs within Japan and overseas
Responses to authorities in each country/area regarding CMC regulatory activities for the conduct of clinical studies
New technology development/acquisition for CMC activities
Message from Head of CMC Research

Toshiro Sakai, Ph.D.
CMC Research was established in April 2022 to accelerate and advance the development of early-stage projects in Astellas. There are well over 10 different types of new modalities being tackled by CMC research. The probability of success of new pharmaceutical product development is not high. Unfortunately, most of the products under development are dropped at the stage before commercialization. Thus, to promote early development projects, it is important to focus on efficiency and speed, evaluate many products under development while making maximum use of limited resources, and identify the possibility of demonstrating Proof of Concept (POC) quickly.
In order to achieve the fastest development with consistent operations, CMC research has an organizational structure much like a small Pharmaceutical Technology division that integrates all functions related to pharmaceutical technology development, such as drug substances, formulations, analysis, manufacturing, and regulatory affairs.
From the stage of drug discovery research, we actively engage the research from the aspect of manufacturing, which is our strength, to improve the efficiency of research in the generation of development candidate products. In addition, since we understand the characteristics of products in the early stage, we accelerate the development of technologies for the start of subsequent clinical studies, and speed up the promotion of projects.
In addition, in the development of products in new modalities, there are many products that cannot be handled using only technologies we have accumulated through our experience with developing new drugs. For this reason, we are taking on the everyday challenge to achieve the fastest development speed similar to that for products with existing modalities through the combination and fusion of advanced manufacturing technologies developed by members with diverse expertise and through joint research with external experts.
We want to deliver valuable pharmaceutical products to patients as soon as possible. To this end, we look forward to working with researchers who will be able to learn, help each other grow and think alongside their peers with diverse specialties, and who will open up new avenues for agility with out-of-the-box thinking.
Introduction (Toyama Technology Center)
Overview
Our plant in Toyama, which has been named the Toyama Technology Center, is a bio-lead hub for Astellas
Pharma, Inc. for which a technology research function was added to the conventional factory
function.
We develop processes for fermentation, antibodies, and bio drug substances in the research and
development stage, as well as scale-up, and manufacture the corresponding formulations. We are also in
charge of commercial production after approval, and we are advancing the development of engineers.
A Message from the Center Director

Koji Nagao
The pharmaceutical industry of Toyama Prefecture is a traditional industry with a history of more than
300 years since the Edo period and, at present, there are approximately 80 pharmaceutical
manufacturers and more than 100 manufacturing sites in the prefecture.
In 1992, the Toyama Technology Center began operations as a manufacturing plant for the drug substance
for "Prograf," an immunosuppressant agent produced using fermentation technology to search for new
bioactive substances from natural products in the pharmaceutical city of Toyama. Subsequently, we
expanded our manufacturing activities to include Prograf capsules, granules, and ointment/packaging.
In addition, we have been manufacturing the drug substance for the antifungal agent "micafungin" since
2001. These products are delivered to patients around the world as global products in collaboration
with the Kerry Plant in Ireland and the Takaoka Plant.
In 2019, the United States Food and Drug Administration (FDA) gave manufacturing approval for PADCEV,
an antibody-drug conjugate approved as a groundbreaking therapeutic drug, and we began commercial
production. Furthermore, Zolbetuximab, an antibody drug following PADCEV, is currently in the global
approval application stage, and in order to perform its commercial production, a new 4-story antibody
drug substance manufacturing facility with a total area of 8,000 m² was constructed, and stared
operation in March 2021.
The Toyama center has been expanding business into the field of antibody drug substance manufacturing,
building on the respective elemental technologies and knowledge of fermentation, purification,
analysis, quality control, GMP, engineering, and environmental control, which originate from microbial
fermentation technology. In the future, we will continue to evolve as a biological manufacturing site
for Astellas, responsible for manufacturing existing products and new modalities.
We manufacture pharmaceutical products that are closely related to patient survival, including those
for transplants, infectious diseases, and cancer. To this end, we believe that our mission is to
continue stable production and supply under any circumstances using the latest technology and the best
teamwork. And we are also working to establish the system capabilites which enables stable production
and supply of pharmaceuticals into the future. At the same time, we aim to be an organization where
each employee can experience growth, a sense of accomplishment, and a sense of challenge, placing the
safety and health of our co-workers first.
Toyama Technology Center is looking for energetic colleagues from various orientations with a variety
of backgrounds. Would you like to shape your dreams and future with us?
Working employees (Toyama Technology Center)
Employee Q&A
Culture Technology Section, Technology Development Section
Background: Department of Applied Animal Science
Year of joining: 2019
Tomoaki Kogo
-
What is your current job description?
In the Technical Development Section, to which I belong, we are engaged in the stable supply of antibody drugs and the examination and improvement of manufacturing procedures at the actual production scale. Currently, I am handling two products: an intermediate product of PADCEV, which is designated as a breakthrough therapy by the FDA, and zolvetuximab, which is in the Phase III clinical trial stage. In my first year with the company, there was an FDA inspection of PADCEV. I was able to experience firsthand the importance of social responsibility and the patients that await us beyond our day-to-day work. By my third year, I was involved in a variety of tasks, including the start-up of a new Astellas manufacturing facility, the introduction of zolvetuximab into the new facility, and the scale-up of PADCEV production as it continued to increase. Now, in my fourth year with the company, I have increased my skills and knowledge necessary for antibody production, and I feel that I am contributing to the stable production of high-quality drugs to deliver them to patients around the world who are waiting for them
-
When do you find work interesting or difficult?
I feel fun and a sense of accomplishment when I face a difficult problem and clear it with a method that has no track record so far. The global COVID-19 epidemic has dramatically changed the environment surrounding pharmaceutical manufacturing, creating many challenges. As a result of tackling one of the issues, we adopted a new policy and solved the issue caused by COVID-19. On top of that, we were able to introduce the best system with lower costs than before. In the process of solving problems, it is essential to collaborate not only with our own department, but also with other departments and outside the company that we usually have little involvement with. However, I still vividly remember the moment when the initiative took shape, I was overwhelmed with joy.
-
In your work, what challenges would you like to take on in the future?
Based on the skills and knowledge I have acquired through the stable production of PADCEV intermediates and the development of commercial manufacturing processes for zolbetuximab drug substance, I will develop and strengthen commercial manufacturing processes for new modalities such as cell therapy. I would like to work as a biopharmaceutical professional.
-
What attracted you to work at Astellas?
The great attraction of working at Astellas is the people. Delivering a single drug to patients involves more people than you might imagine, including research, development, manufacturing, and sales departments. In order to deliver drugs to patients more quickly, collaboration with various people is essential. I empathized with the atmosphere that the senior who was in charge of the company information session introduced the workplace lively, and I wanted to work at Astellas. When I actually joined the company, that intuition was correct. Of course, I had the cooperation of everyone in other departments when I was solving the aforementioned issues, but I am also very grateful to my boss, who encouraged me to grow while maintaining an appropriate sense of distance and correcting my trajectory as necessary. I'm here.
A day in the life of Taku Shibuya
| 8:00-9:00 | Radio gymnastics. At the morning meeting, we share the flow of work for the day, the division of roles, and the work status up to the previous day. |
| 9:00-12:30 | manufacturing work. |
| 12:30-13:30 | Lunch in a buffet style dining room. There is a seasonal local menu! |
| 13:30-15:00 | Continue manufacturing work. Clean up after work. |
| 15:00-16:30 | desk work. Confirmation of records used in manufacturing and preparation of documents such as procedure manuals. |
| 16:30-17:00 | Prepare documents and equipment to be used in the next day's manufacturing work, check the work flow, and go home. * Affiliation at the time of posting. |
* Affiliation at the time of posting.
Working place (Toyama Technology Center)
Introduce my workplace!

Shohei Takaki
Downstream Technology, Technology Development
Our role in purification technology involves antibody manufacturing and launching investigational new drugs (IND), and subsequent stable production continuously. When scaling up from laboratories to industrial facilities, we consider and determine which operational parameters of the facilities and equipment are suitable for production. Building a stable supply system with our suppliers of raw materials is also one of our important duties. To prepare a product for the market, we perform various tasks to obtain approval for drugs, such as collecting the data required for drug application. In particular, for the biopharmaceutical approved by the FDA in 2019 and designated as breakthrough therapy, we had to minimize the lead time to market the product. It was definitely a challenge, but I was delighted that I could contribute to achieving our goal of “swiftly delivering new drugs to patients”, and it reminded me of the rewarding aspect of my job. To ensure the stable production of new drugs after their launching, we continually strive for improvement to build a robust and efficient manufacturing system.
Location Introduction
Toyama has plenty of delicious seasonal foods, such as firefly squids and shiro ebi shrimp in the spring, iwagaki oysters in summer, pears in autumn, and Japanese amberjack in winter, and enjoying the gourmet cuisine of each season is one of the attractions of the prefecture. Our facility is located in the city of Toyama where there are plenty of restaurants and shopping malls, making it a convenient place to live. I think Toyama is an ideal environment for outdoorsy people to stretch their legs, as it’s easy to access the beach, mountains, and rivers. Although it snows during winter, you don’t have to worry too much as plenty of winter weather supplies are available to put you through it. Furthermore, the snow in the mountains provides us with fresh water, a beautiful natural environment, the joy of winter sports, and many other blessings of nature.



Introducing the Workplace
Toyama Technology Center is located close to the “light rail”, a streetcar, station that connects Toyama Station and Iwasehama Station. We have around 400 employees at our center, and our duties include manufacture of biopharmaceuticals and fermentation-derived drug substances and products. Quality control and quality assurance for manufacturing are also part of our duties. The roof of our administration building is in a unique shape depicting “Owaragasa”, a traditional Japanese hat worn by dancers in Yatsuo City’s local performing arts festival called “Kaze-no-Bon”. On sunny days, you also get to see the majestic view of the Tateyama Mountains through the window. You can make your break time extra enjoyable by having lunch in the cafeteria, which offers a variety of food choices, and playing sports at the gym with your colleagues after work.



Introduction (Takaoka Plant)
Overview
The Takaoka Plant specializes in manufacturing of injection products.
We have
several capability to manufacture injection products and continue to evolve in line with the times as a
base for the development of injection products.
Although the requirement of regulatory authorities in
various countries become more stringent, we always stay one step ahead to improve the level of quality
control and, in particular, we pursue the advancement of aseptic control technology and the action
agaist contamination of foreign matter, which are essential for the manufacture of injection
products.
Based on the technologies we have cultivated over many years, we deliver commercial
pharmaceutical products to more than 50 countries over the world, while also focusing on the launch and
manufacture of new products.
Moreover, in 2018, we began manufacturing of injection products for
clinical trials, supporting the development of new pharmaceutical products at Astellas.
In addition
to our main work in manufacturing of injection products, we also provide technical expertise to
laboratories and other facilities that require aseptic control, serving as a training base for engineers
in this field where demand is rapidly increasing.
A Message from the Plant Director

Masafumi Dohi
The time has come where research and development on a variety of pharmaceutical products that can be
new therapeutic agents for intractable diseases for which there are no effective treatment methods
using biotechnology is advancing.
Many of these are macromolecules or even higher-dimensional cells, and thus, various dosage forms are
assumed; however, what remains unchanged now from the past is that pharmaceutical products need to
reach the body safely.
Almost of biopharmaceutical products are injection type which definately require the aseptic control
technology to to prevent contamination with bacteria.
However, there is difficulty to perform aseptic filtration due to the size of the main body of the
biopharmaceutical product.
The mission of aseptic control is to solve the issue of how to ensure the sterility of such
products.
Also, we have to take care for avoiding contamination from foreign matter for injection
products.
In order to contribute to the health of people around the world through innovative and reliable
pharmaceutical products, we are constantly striving to achieve higher levels of aseptic control.
With your diverse expertise and the aseptic control technology that we have cultivated at the Takaoka
Plant and Astellas, let us usher in a new era.
Working employees (Takaoka Plant)
Employee Q&A

We improve the aseptic control necessary for the production of injectable pharmaceutical products on a daily basis, manufacture investigational products, and establish manufacturing for new pharmaceutical products.
Pharmaceutical (Sterile) Manufacturing Engineering
Graduated from the
Department of Pharmacy
Joined the company in 2019
Megumi Tamura
-
What is your current job description?
We improve the aseptic control necessary for the production of injectable pharmaceutical products on a daily basis, manufacture investigational products, and establish manufacturing for new pharmaceutical products.
I am in the investigational product department of the plant and am involved in a project to establish manufacturing for a new product.
Recently, I have been investigating the sterilization of various materials necessary for the production of new products in the project to establish manufacturing for a new pharmaceutical product. For example, in order to determine the sterilization conditions for rubber stoppers, the first task is to verify whether sterilization can be performed with the same operational program as before, and to verify whether there is any change in the quality of rubber stoppers before and after sterilization. Since the establishment of manufacturing for a new pharmaceutical product will be the basis of manufacturing that will continue for many years to come, it is necessary to examine this from a more fundamental and broader perspective, such as whether existing processes and process control values were appropriate in the first place, even if the results were within acceptable limits. Also, once a procedure is established, it is difficult to change it; thus, one of the tasks is to identify issues as much as possible and propose improvements at the evaluation stage.
It is also important to comply with the increasingly stringent regulatory requirements of each country. For example, although regulations for data integrity (established to prevent falsification or forgery of data) have become more stringent recently, it is not practical to keep updating/upgrading all manufacturing equipment, even if these regulations can be easily met through updates of the manufacturing equipment. Thus, we must consider how we can make up for the portion that cannot be covered by hardware (equipment) with software (operational solutions), and what we can do to minimize the burden on the field. This requires both knowledge of GMP and a perspective as an operator. Currently, I am working on the establishment of operational procedures of a data collection system to ensure a sterile environment while receiving advice from my seniors. -
When do you find work interesting or difficult?
When I am elucidating the entire view of aseptic control
Sterile products, by definition of the term, contain "0" bacteria. However, because bacteria are not visible to the naked eye and can be found anywhere, it is not easy to accomplish this task every day. At the Takaoka Plant, we produce tens of thousands of sterile products every day. In order to continue to ensure that no bacteria exist even when any one of these products is used, many people have accumulated know-how over a long period of time.
Antibodies and cells, which are the core products currently being developed, cannot be sterilized by methods such as heating because if stress is applied, the protein will degenerate, and the medicinal effect will be lost. Thus, in order to ensure the sterility of the products, strict sterility control is applied throughout the manufacturing process. In other words, each process is thoroughly controlled, from environmental control at manufacturing sites to the cleanliness level of the instruments and piping that directly come into contact with the drug solution before filtration (process control). In addition, we periodically verify that the performance of each facility is appropriate (validation). Since there are no means to verify from the outside whether the finished product is sterile or not, we have no choice but to ensure that the entire process is aseptically controlled in this way without any gaps.
This know-how was obtained through a combination of various disciplines, technologies, and ingenuity, rather than something established through university lectures. When I joined the company, I felt that these things were intricately intertwined strings, but when I gradually untied the strings and gained essential knowledge, and when I was able to systematize and understand them, I began to see what was fascinating about aseptic control. -
In your work, what challenges would you like to take on in the future?
I want to take part in the launch of a new plant as an aseptic control engineer that can meet global standards.
At present, the core products under development are biopharmaceuticals including antibodies and cells, and we need a factory equipped with aseptic control technology to deliver these products to the world. When starting new plants, we do not simply copy the structure of the Takaoka Plant; we are required to build a better one in light of issues specific to the products being manufactured and the latest regulatory trends. In order to be able to carry out these tasks, we must not just perform our daily tasks, but we must also always be thinking about and considering the rationale for setting process control values, compliance with guidelines, as well as the reason and necessity for conducting the tasks. Although I am still studying now, I hope to be able to play an active and lead role as an expert in aseptic control in any location.
Also, if you can speak English, you will be able to play an active role in the future by transferring technology to overseas sites, responding to inspections, and catching up on the latest regulatory requirements. Fortunately, the Takaoka Plant is actively implementing initiatives with an awareness of globalization. Every day during lunch break, volunteers participate in English discussions, hold monthly information exchange meetings with overseas plants, and there is an English newspaper placed in the cafeteria. In these ways, we are creating an environment where people can enjoy English every day. By making the best use of such an environment, I aim to be able to produce high results in my work while manipulating English as if it were Japanese.
With the rapid increase in the development of biopharmaceuticals, I feel that the need for aseptic control engineers in the company is very high and, even for challenges that may be slightly out of reach, my seniors and supervisors are pushing me forward. -
What is the appeal of working at Astellas?
I can contribute to providing patients with exciting and attractive new pharmaceutical products.
Astellas is a leading manufacturer of new pharmaceutical products in Japan. One of our greatest strengths is our continuous focus on new pharmaceutical products. One of the things that surprised me when I joined the company was how many people were involved in the process of making a single pharmaceutical product. Excellent researchers from various fields, such as researchers in the Pharmaceutical Research and Technology Labs, engineers who until recently were here participating in the training, and those I met in the formulation forum and analysis forum during my first year, etc., combine their wisdom to create a new pharmaceutical product. That alone is not an easy task. However, these products cannot be delivered to patients without aseptic control technology. My job is to go through this exactly, and I can always feel that the pharmaceutical products we make may change the future for patients.
I am in charge of investigational products, and new attractive drugs that I myself would be excited about are coming one after another. There are patients waiting for these. We can contribute to that, and that is the attraction of working at Astellas.
A day in the life of Megumi Tamura
| 7:00 | Get up. It takes 10 minutes by car to get from my home to the office. |
| 8:00 to 8:05 | Get to work. Change clothes. |
| 8:05 to 8:30 | Morning assembly. Radio calisthenics. Check e-mails. |
| 8:30 to 11:00 | Conduct a verification test for sterilization conditions. |
| 11:00 to 12:00 | Discuss the results of yesterday's verification test with my seniors. |
| 12:00 to 13:00 | After lunch at the cafeteria, participate in an English study meeting (debate) with fellow volunteers. |
| 13:00 to 14:30 | Conduct the verification test for sterilization conditions (continued). Check e-mails in between. |
| 14:30 to 16:00 | Prepare reports and materials. |
| 16:00 to 17:00 | Meetings. Remote meetings may be held with overseas plants. |
| 17:00 to 18:00 | Prepare for the verification test tomorrow and prepare meeting minutes. |
| 18:00 onwards | Leave the office (the time varies depending on the day. I may leave the office earlier by using the flextime system). On weekends, I cook and read foreign books. |
* Affiliation at the time of posting.
Working place (Takaoka Plant)
Introduce my workplace!

Narumi Ota
Production Technology Section
Parenteral Production Technology Development
Toyama is known as the “Capital of Medicine”. I joined Astellas because I thought I’d like to work in
a pharmaceutical industry in my hometown. I’m currently assigned to the manufacturing sector for
investigational new drugs (IND), where I work as an operator in the sterilization process and the line
leader for inspection and packaging. INDs differ from commercial products in that they require
different drug substances, excipients, and packaging materials, as well as having different
manufacturing conditions depending on the project. Therefore, IND production requires us to review
manufacturing methods in advance, create documents on manufacturing procedures and conditions, and
gather information on raw materials and packaging materials.
Although it can be challenging to
fully execute a broad range of tasks to produce an IND within the deadline, I find my work very
rewarding as I can improve my skills through proactive collaboration with internal and external staff.
Location Introduction
With over 400 years of history, Takaoka has numerous historical landmarks, traditional crafts, and festivals. The Tanabata festival is one of Takaoka's summer traditions and is held every year near our facility. I always enjoy eating street food on my way home while strolling through the town colorfully decorated with Tanabata ornaments. The city is also rich in nature, with mountains to the west and Toyama Bay to the northeast, offering plenty of picturesque scenery around the city. Many people visit the “Amaharashi Coast” with cameras in hand to see the Tateyama Mountain range overlooking Toyama Bay. My favorite passtime is relaxing at a nearby café while enjoying the view of the Sea of Japan.



Introducing the Workplace
Takaoka Plant began operating in 1975 as the manufacturing base for antibiotic capsules and fine granule oral drugs. Today, the plant manufactures sterile formulations and packaging for injectable drugs. The cherry blossom trees planted around the perimeter of the facility are breathtaking when they’re in full bloom. The plant also has excellent views, so we can enjoy our lunch while looking at the Tateyama Mountains from the large cafeteria window on fine days. Although most of our staff drive to work, our facility is also accessible by public transport as it’s only a 15-minute walk from Toide Station.



Introduction (Takahagi Technology Center)
The Takahagi Technology Center began operations in 1974 as a pharmaceutical drug
substance plant, and is situated in Takahagi City in the northeastern part of Ibaraki Prefecture. With
the subsequent relocation of synthesis technology research and developmental drug substance
manufacturing facilities to the Takahagi site, the Center began development of manufacturing and testing
methods in conjunction with investigational drug substance production. To date, the Center has launched
production of many new products including Gaster, Perdipine, Harnal, Bonoteo, Vesicare, Irribow, and
Betanis.
As Astellas Group's leading facility for drug synthesis, the Takahagi Technology Center
works closely with the Pharmaceutical Science & Technology Labs, Chemical & Biological Technology Labs,
and Analytical Research Labs to constantly improve its technical capabilities with a strong sense of
mission to expedite delivery of new drugs that patients need them, and to facilitate the supply of
high-quality pharmaceuticals to the global market.
A Message from the Plant Director

Nobuo Kasahara
Since commencing operations in 1974, the Takahagi Technology Center has supplied
more than 30 commercial drug substances and numerous developmental-phase investigational drug
substances. As a core Astellas facility for synthetic drug substances, the Center coordinates closely
with our research laboratories to play an important role in the development of processes and test
methods for new drug candidates, the seamless launch and stable supply of new products, and the
transfer of technology to other sites, including the Group's plant in Ireland. Our facility also
features containment equipment for large-scale synthesis of anticancer drugs and other highly
pharmacologically active substances. Leveraging these capabilities, we are striving to synthesize a
broad range of compounds with the aim of expediting the delivery of new drugs that patients need
them.
Located in the northern part of Ibaraki Prefecture, Takahagi City is surrounded by both
sea and mountains. Amidst this rich natural environment, our employees are always dedicated to
patients, working to improve our technical capacity and enhance our operating quality. We will
continue to deliver high-quality, value-added pharmaceutical products to patients while ensuring
stable supply in harmony with the natural environment.
Working employees (Takahagi Technology Center)
Employee Q&A

I am responsible for process development for manufacturing investigational drug substances and for improving efficiency and troubleshooting issues in drug substance manufacturing, and am working to ensure stable supply and to maximize the value of drug substances.
Synthetic Drug Substance Manufacturing, Masters in Drug Discovery
Science,
joined the company in 2014
Taiga Ota
-
What is your current job description?
I am involved in the development and improvement of manufacturing processes and the validation of efficacy for investigational drug substances, as well as stable drug substance supply.
The Takahagi Technology Center is a core facility for drug substance manufacturing functions focusing on chemical synthesis, and plays a key role in realizing the stable supply of chemically-synthesized drug substances. Within this environment, Production Technology 1 where I work collaborates with the Pharmaceutical Science & Technology Labs, Chemical & Biological Technology Labs.(CBTL) and others in the development of manufacturing processes for investigational drug substances and the verification of the effectiveness of these processes using pilot plants, and in the supply of manufactured investigational drug substances for toxicologic evaluation and clinical studies in order to realize just-in-time and continuous delivery of new drugs to those who need them.
In recent years, as drug development has accelerated, there have been more opportunities for our engineers to work closely with laboratories in developing processes that can realize greater efficiency. However, in the manufacture of developmental-stage investigational drug substances, information on physicochemical and bioactive properties is often unavailable, so it is not uncommon for previously unobserved phenomena to occur for the first time during manufacturing. To prevent these kinds of unforeseen issues, it is essential to obtain various data in advance from myriad perspectives during process development, including verification tailored to the equipment being used based on chemical engineering and equipment engineering methodologies, and verification based on operability considerations. Furthermore, by providing feedback of this information, including knowledge gained during manufacturing, to each laboratory and person in charge of manufacturing, we can contribute to the development of efficient manufacturing processes. As a result, environmental considerations, safety measures, quality maintenance, and cost reductions in all manufacturing operations are realized, all of which lead to an early and stable supply of the drug to patients who are waiting for it.
That is why I feel that the work of Manufacturing Section 1 is very rewarding, and allows me to contribute to the creation of significant value by leveraging various knowledge and technologies. -
When do you find work interesting or difficult?
I find it fascinating to perform validation in order to solve problems, and to work towards improving quality and operating efficiency.
In the manufacture of investigational drug substances in particular, scalable outcomes are scarce and data is often insufficient so it is important to identify potentially challenging operations through preliminary validation, and to adopt effective countermeasures. I feel a major sense of achievement when these initiatives for mitigation contribute to process stability and efficiency. The fact that things do not always go as expected is a challenge, but I find this job interesting in that we are constantly working towards solutions through trial and error. The manufacturing of investigational drug substances also involves not only my own department but many other related departments, including the research centers and laboratories. I think the appeal of this job is that a wide variety of people from different backgrounds share their experience, knowledge and skills, and work together to solve common problems.
-
In your work, what challenges would you like to take on in the future?
I would like to further develop my capabilities related to the manufacture of drug substances based on chemical synthesis.
As I mentioned in my response to the Q1, the relationship between each laboratory and manufacturing site has become closer in recent years. This closer collaboration has made it more important for those of us involved in production to adopt a research perspective as well as a manufacturing perspective. By considering and recognizing the implications of operations involved in manufacturing processes, we can devise and develop more effective methods. I believe that the best human resources are those capable of contributing to the creation of manufacturing processes not from a single perspective, but rather from various perspectives including the environment, safety, quality, delivery, and cost, without having to compromise on what’s important. It is also crucial to access new technologies to strengthen our drug substance production capabilities. In addition to the manufacturing of small molecule drug substance, I would like to expand my potential by learning more about technologies in chemical synthesis and other fields.
-
What is the appeal of working at Astellas?
The appeal of working at Astellas is that it provides an environment where I can proactively pursue new challenges.
Although the pharmaceutical industry environment and the needs of the public change on a daily basis, Astellas' mission to constantly deliver valuable products to patients remains unchanged.
Astellas has recently been expanding its efforts not only in pharmaceuticals but also in non-pharmaceuticals (Rx+) with the aim of contributing to patients' physical and mental health. As a company, we are therefore flexibly geared towards the challenge of developing new areas, and as an individual employee, we are given a range of options on how to tackle this challenge. I think the appeal of Astellas is being in an environment where I can think about what I need to do and then pursue challenges in various fields that both leverage my abilities and hone my potential.
A day in the life of OTA
| 7:00 | Wake up (10-minute drive from home to office) |
| 8:15 to 8:20 | Check emails and schedule for the day |
| 8:20 to 8:30 | Radio calisthenics, morning meeting |
| 8:30 to 12:00 | Prepare and perform tests, prepare documents, attend meetings (may vary according to work schedule) |
| 12:00 to 13:00 | Lunch (company cafeteria), lunch break |
| 13:00 to 16:30 | Continue tests, prepare documents, attend meetings (may vary according to work schedule) |
| 16:30 to 17:15 | Pack up, prepare for next day |
| 17:15 | Leave the office (may vary according to work schedule) |
* Affiliation at the time of posting.
Working place (Takahagi Technology Center)
Introduce my workplace!

Hiroki Kawanaka
Quality Control Department Quality Control Unit 1
Quality Control Unit 1, which I belong to, conducts all kinds of quality tests related to investigational drug substances. As there is less experience in manufacturing investigational drug substances than there is in commercial drug substances, it is not unusual for troubles to occur. Therefore, we preliminarily assess the risk of troubles carefully, then the identified risks are mitigated in advance to carry out quality tests smoothly during actual manufacturing. To supply products just in time, establishment of robust testing methods is essential. Quality Control Unit 1 also develops raw material testing methods by investigating target compounds and finding the most optimized testing equipment and conditions. It does not always proceed in accordance with the original plan; however, I am really interested in communicating with related department members and making an effort to solve problems with them. In addition, Quality Control Unit 1 contributes to effective manufacturing process development by providing feedback of the data obtained from manufacturing to the laboritories.
Location Introduction
Takahagi developed as a castle town in the Edo period when Matsuoka Castle was built. The ruins of Matsuoka Castle are notable for their beautiful cherry blossoms, and the nearby Oyashiki-Dori lets you experience the ambiance of the traditional castle town. Takahagi initially developed as a city of coal industry following the opening of the Joban Line in the Meiji period, and is now evolving into an industrial town due to the expansion of businesses into industrial parks. The allure of Takahagi is that you can experience various outdoor activities due to its rich natural surroundings; you can enjoy swimming, surfing, and fishing at the beach, and golfing and lake canoeing in the mountains. Hananuki Gorge, Hitachi Seaside Park, and Hagi Village are definitely worth visiting. From season to season, you’ll enjoy sports, camping, and gourmet food in the magnificent nature of Takahagi.



Introducing the Workplace
The extensive premises of Takahagi Technology Center are around three times larger than Tokyo Dome and located 4 kilometers from Takahagi Station. Since the facility is located on a slighly elevated site overlooking the ocean, there are ocean views as far as the eye can see. An ancient grave known as “kofun” situated near the premises also adds an extra uniqueness to the center. The primary function of Takahagi Technology Center is drug substance manufacturing functions focusing on chemical synthesis, making the center a vital base for the stable supply of chemically-synthesized drug substances. The facility also carries out various other duties in addition to manufacturing, including lab experiments and examinations, quality tests and assurance, environment, safety, and health (ESH) management, and worksite operations. Collaboration with each of these departments contributes to the stable supply of pharmaceutical products. Although we have fewer staff members compared to our other business facilities, our small size makes it easier for us to work as a team, and many of us actively participate in club activities.



Introduction (Yaizu Technology Center)
The Yaizu Technology Center is the main plant for solid preparations. In FY2022, we launched an injectables production facility and will also commenced the production of continually-advancing biopharmaceuticals. Through tightknit collaboration with the Formulation Research Center, the Center has already succeeded in bringing many new products to market. We have committed to improving our formulation technologies and manufacturing equipment with the aim of achieving high-quality production and cost reductions through automation.
A Message from the Plant Director

Tomohiro Yoshita
The Yaizu Technology Center has served as the core manufacturing site for the Astellas Group’s drug products. In 2023 we marked our 55th year in operation, during which time we have manufactured more than 100 different pharmaceutical products delivered to patients around the world.
In addition to commercial products, we are also responsible for manufacturing development-phase investigational medicines. Through close coordination with our onsite Formulation Research Center and Materials Research Center, we are expeditiously working to deepen our understanding of developmental products and contribute to speeding up the development process in order to facilitate rapid delivery of new pharmaceutical products to patients.
Featuring both investigational drug and production line equipment with containment functions, the Yaizu center is responsible for developing and commercializing anticancer drugs and other highly pharmacologically active compounds, and has a proven track record of commercializing a wide range of formulations.
In FY2022, we launched a new production facility for injectable drugs, and will also commenced production of biopharmaceuticals, which have been actively developed in recent years.
As the level of quality demanded by regulatory authorities around the world increases, it is essential to foster a culture capable of maintaining and improving that quality. The Yaizu Technology Center is striving to foster this ‘quality culture’ while consolidating more robust quality systems leveraging our experience of inspections by overseas authorities due to our development of pharmaceutical products on a global scale.
Yaizu City is a comfortable place to live with an amenable climate. We have taken proactive initiatives to coexist with the community, contribute positively to the region, and address environmental issues in order to ensure that we can continue providing pharmaceutical products from this comfortable environment. By evolving our strengths in commercial product integration honed over more than half a century of new product development, the Yaizu Technology Center will continue the stable supply of high-quality products to patients.
Working employees (Yaizu Technology Center)
Employee Q&A

Master of Bioresource Sciences
Joined the company in 2018
Yoshie Maeda
-
What is your current job description?
To contribute to the stable supply of safe and reliable products by expeditiously and reliably conducting studies and ensuring quality based on accurate data.
Yaizu Technology Center’s Quality Control Department has a section dedicated to quality testing of commercial and investigational drug raw materials (i.e., drug substances, excipients, and packaging materials), microorganisms, and formulations.
I am mainly in charge of drug substance testing using high-performance liquid chromatographs, gas chromatographs, infrared spectrophotometers, and other analytical instruments to qualitatively and quantitatively assess their active ingredients and check for impurities, and to ensure they conform to drug manufacturing specifications using accurate data. I contribute to the stable supply of high-quality pharmaceutical products by completing tests accurately and quickly so as not to affect the manufacturing schedule.
I am also part of an interdepartmental Foreign Matter Analysis Team. Our analyses cover all pharmaceutical products, investigational drugs, raw materials, and formulations. They include foreign substances found in raw materials or generated in manufacturing processes, and those related to product complaints from healthcare providers, such as analyzing broken tablets. Contamination by foreign matter is strictly prohibited in pharmaceuticals on which patients’ lives depend. To ensure the continued delivery of safe and reliable pharmaceuticals to patients, we strive to reduce the risk of contamination by expeditiously performing analyses, identifying the cause of contamination, and working with the Manufacturing Department and other relevant departments. The work of the Foreign Matter Analysis Team also allows me to learn about topics other than raw materials so it has helped broaden my knowledge and perspectives, which in turn gives me a feeling of personal development every day.
Since starting this job, I have realized the importance of such awareness.
Even if our test results fall within the reference range, it is essential to notice anything unusual and to explore the potential causes of anomalies from various perspectives. When analyzing foreign matter, it is crucial to notice and estimate potential sources of foreign matter risks based on identified elements. We are constantly noticing and investigating these risks on a daily basis, and we implement the necessary improvements to achieve even higher levels of quality control.
All of our work involves a great deal of responsibility. We strive to realize ever-higher levels of quality control, and engage in our work with a genuine sense of urgency and responsibility. I feel that my work contributes to Astellas' drug manufacturing, which is very rewarding in itself. -
When do you find work interesting or difficult?
When I’m challenging myself to learn analytical techniques for new drug substances.
At the Yaizu Technology Center, new products have been launched every year since I joined the company, and I have been involved in the quality testing of several new drug substances. To perform quality testing of new drug substances at the Yaizu Technology Center, analytical technology transfers are needed to acquire the necessary analytical technology from laboratories with relevant expertise or from drug substance manufacturing sites outside Japan. Many things don’t go smoothly or are uncertain when we study a new test method for the first time. We overcome these issues and uncertainties by consulting with our experienced senior staff and by working closely with laboratory personnel to gather the information needed to facilitate future testing. The process of solving problems one by one is interesting despite the inherent difficulties because it allows me to expand my knowledge of testing methods and analytical equipment. What’s more, solving problems in an ad hoc manner is certainly not ideal for ensuring the stable operation of quality testing in compliance with GMP. It is crucial to implement systems that guarantee a consistent testing environment in which all analysts can perform tests with the same degree of precision and without error, regardless of when or under what circumstances the test is performed. I feel challenged on a daily basis, not only in terms of my skills and knowledge of testing methods and equipment, but also because I need to a flash of idea as an analyst and to be flexible in my ideas; however, I find it interesting.
-
what challenges would you like to take on in the future?
I would like to try analyzing new fields such as antibody drugs and biopharmaceuticals.
To date, the Yaizu Technology Center has been manufacturing pharmaceuticals, primarily solid preparations (small molecule drugs), but we have now started manufacturing antibody drugs. I was fortunately involved in the transfer of analytical technologies for antibody drugs. Because antibody drugs have entirely different properties and characteristics from those of small molecule drugs, we have to perform tests using new equipment that we have never handled before and there are many things that we cannot understand using our existing expertise and technologies in the analysis of small molecule drugs. I hope to learn more about antibody drugs, various analytical techniques, and equipment so as to minimize the potential unknowns that we may encounter. I would also like to further my knowledge of various national regulations and contribute to developing GMP-compliant systems that will ensure higher quality control levels. In the future, I intend to challenge myself to become a more capable analyst not only of small molecules and antibody drugs, but also new biopharmaceuticals, with the aim of developing the analytical skills and knowledge required to handle a wide range of drugs.
-
What is the appeal of working at Astellas?
I feel the most attractive point is that I can continue to contribute to patients around the world who are waiting for medicines.
Astellas is a company that continues to pursue challenges in the development of new drugs. We are focused not only on our specialist fields but also on research and development of advanced and cutting-edge drugs targeting viruses and cells. This approach allows me to feel involved in the future development of various new drugs even while working in manufacturing, which is the most appealing aspect of the job.
Although it doesn’t directly result in the creation of new drugs, quality control work is vital for ensuring that the highest quality drugs are delivered to patients as expeditiously as possible. This is a rewarding job where I can play a part in constantly delivering safe and reliable medicines to patients around the world through drug quality assurance based on accurate data.
Astellas also has many departments covering research, medical & development, pharmaceutical technology, engineering, manufacturing, and marketing. These different departments handle everything from drug research and development to manufacturing and sales. I believe that the appeal of Astellas is that the various departments within the company allow us to closely collaborate efficiently to ensure the stable supply of medicines. The Yaizu Technology Center where I work comprises not only the plant but also the Formulation Research Center and Materials Research Center on the same site, and this layout facilitates interdepartmental communication. By sharing opinions from various perspectives and working closely together, we are able to manufacture investigational drugs, launch new products quickly, and ensure a stable supply of existing products.
A day in the life of Yoshie Maeda
| 8:15 | Arrive at office, check email, participate in radio calisthenics |
| 8:25 | Team meeting, training |
| 8:40 | Review analytical data from previous day |
| 9:00 | Testing or sampling |
| 12:00 | Lunch |
| 13:00 | Continue tests (prepare documents in between testing) or attend meetings |
| 16:00 | Check and summarize analytical data for the day, pack up |
| 17:00 | Prepare testing for the next day and check schedule |
| 17:15 | Return home |
* Affiliation at the time of posting.
Working place (Yaizu Technology Center)
Introduce my workplace!

Kazuyoshi Chida
Production Technology Department 3 Technology Development Unit
I joined Astellas because I wanted to work at a global company. I decided to apply for a manufacturing-related position because I was fascinated by the idea of creating and delivering products directly to patients. In my first year at Astellas, I was assigned to the department of commercial pharmaceutical product production. I was transferred to the department of investigational new drug (IND) production in my second year, where I performed duties specific to the phases of clinical trials for about five years. I’m now in the Technology Development Unit, where I work on a wide variety of production-related tasks. Some of my duties include collecting data related to product quality and production, examining quality improvement and issue resolution, and transferring technology to enable other plants to produce the products manufactured at our facility. I find it rewarding to work in this ever-changing environment where I face such challenges as implementing new approaches and technologies, dealing with the latest regulations, and establishing global connections.
Location Introduction
Gourmet cuisine is definitely one of the best features of Yaizu. The city is abundant with fresh,
delicious seafood such as eel, sakura shrimp, red bream, tuna, and bonito. Yaizu also has numerous
historical sites, including places associated with Ieyasu Tokugawa and the old Tōkaidō road. It’s also a
comfortable place to live due to the mild climate with no snowfall in the winter. Since the town is
surrounded by mountains and sea, you can enjoy outdoor activities like mountain climbing, camping,
fishing, and motorbike touring. In addition to offering easy access to bullet train stations and
freeways, our location is also near Mt. Fuji Shizuoka Airport, making the area convenient for
long-distance travel.

Introducing the Workplace
Yaizu Technology Center is located in the urban district within walking distance to Nishi-Yaizu Station, allowing convenient access for employees. The center is responsible for the research and development (R&D), production, and quality control of products such as solid and injectable drug preparations. Over the years, Yaizu Technology Center has launched a large number of new products worldwide. We have around 800 employees, including staff members from our partner company, and many of them seem to be gentle and calm individuals. Our workplace allows us all, from new employees to veterans, to make the most of our indiviuality and use it to succeed in our jobs. In terms of the facilities, our massive premises include tennis courts, fields, and several new office buildings. You can also get excellent food from the cafeteria, which sometimes offers special event dishes besides the usual menu. The row of cherry blossom trees planted at the main entrance is one of my favorite spots at Yaizu Technology Center.


Work Location
-
Tsukuba Research Center (Ibaraki, Japan)
Access Map -
Tsukuba Biotechnology Research Center・Tsukuba Tokodai Technology Center (Ibaraki, Japan)
Access Map -
Takahagi Technology Center (Ibaraki, Japan)
Access Map -
Yaizu Pharmaceutical Research Center・Yaizu Technology Center (Shizuoka, Japan)
Access Map -
Toyama Technology Center (Toyama, Japan)
Access Map
*Placement varies depending on the job type.






